
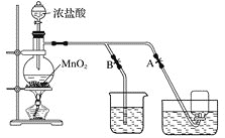
����Ŀ��ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣
��֪��Na2SO3+H2SO4��Na2SO4+SO2��+H2O

�ش��������⣺
��1��װ��A��ʢ��Ũ���������������____________��
��2��װ��B��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ�����������____________��
��3��װ��C�б�����SO2��____________�ԣ�װ��D�б�����SO2��____________�ԣ�װ��D�з�����Ӧ�Ļ�ѧ����ʽΪ____________��
��4��Fװ�õ�������____________��©����������____________��
��5��E�в�����ɫ�������ð�ɫ�����Ļ�ѧ�ɷ�Ϊ____________�����ţ���ͬ��,���ʵ��֤������ж�____________��
A.BaSO3 B.BaSO4 C.BaSO3��BaSO4
��6������úȼ�ղ�����������ֱ���ŷŵ������У���������Ҫ����������____________��
A.����ЧӦ B.���� C.�۳���Ⱦ D.ˮ�帻Ӫ����
��ҵ��Ϊʵ��ȼú����ͨ������ʯ��ʯ�õ���ʯ�ң�����ʯ��Ϊ���������������SO2��Ӧ�Ӷ�����̶������������������ϡ�д�����н���̶��Ļ�ѧ����ʽ��____________��
���𰸡���Һ©�� ͨSO2Ʒ������ɫ���رշ�Һ©������ֹͣͨSO2������Ʒ����Һ����Һ�ָ���ԭ������ɫ ��ԭ���ԣ� �������ԣ� 2H2S+SO2=3S��+2H2O ����δ��Ӧ���SO2 ������ B ���ð�ɫ��������ϡ���ᣬ�������ܽ� ABC 2CaO+2SO2+O2=2CaSO4
��������
��ʵ��Ŀ��̽��SO2�����ʣ�����ͨ��Aװ���Ʊ�SO2��������SO2ͨ��Ʒ����Һ��Ʒ����ɫ֤��SO2����Ư���ԣ���Ϊ����Ư�ײ��ȶ����رշ�Һ©���Ļ���ֹͣͨ�����壬����ʢ����ɫ��Ʒ���Թܣ�Ʒ��ָ�ԭ������ɫ��֤����Ư���ǻ���Ư�ף����ȶ���ͨ�����Ը�����أ���ɫ��ɫ���������·�Ӧ��5SO2+2MnO4��+2H2O=2Mn2++5SO42��+ 4H+��֤��SO2���л�ԭ�ԡ�ͨ��H2S��Һ�����ֵ���ɫ���ǣ�2H2S+SO2=3S��+2H2O���÷�Ӧ����SO2���������ԡ�ͨ�����ᱵˮ��Һ�����������ˮ��Һ�����ԣ�NO3����H+������ǿ�����ԣ���������SO2�Ӷ�������ɫ�������ᱵ������õ��õ�©����ֹ������������������Һ����β�����ա�
��1��װ��A��ʢ��Ũ��������������Ƿ�Һ©����
��2��������SO2ͨ��Ʒ����Һ��Ʒ����ɫ֤��SO2����Ư���ԣ��رշ�Һ©���Ļ���ֹͣͨ�����壬����ʢ����ɫ��Ʒ���Թܣ�Ʒ��ָ�ԭ������ɫ��֤����Ư���ǻ���Ư�ף����ȶ���
��3�����ݷ�����װ��C�б�����SO2�Ļ�ԭ�ԣ�װ��D�б�����SO2�������ԣ�װ��D������Ӧ�Ļ�ѧ����ʽΪ2H2S+SO2=3S��+2H2O��
��4�����ݷ���Fװ�õ�����������δ��Ӧ���SO2��©���������Ƿ�������
��5�����ݷ�����E������ɫ����BaSO4���������ʵ�飺���ð�ɫ��������ϡ���ᣬ�������ܽ⣬������ʵ������֤��ֻ��BaSO4����BaSO3��
��6������úȼ�ղ����������к��д����Ķ�����̼����������ͷ۳�����������Ҫ��������������ЧӦ�����ꡢ�۳���Ⱦ��ˮ�帻Ӫ������������ˮ����ҵ��ˮ�ȵ��ŷŵ��µ���Ԫ�س��꣬��ѡABC������ʹ�õ��͵ĸɷ���������ѧ��Ӧԭ��Ϊ��2CaO+2SO2+O2= 2CaSO4��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С�鰴����·�ߺϳ���ζ����˹���� ��
��

��֪���ٷ��㻯����A�ܷ���������Ӧ���˴Ź���������ʾ��5�ֲ�ͬ��ѧ��������ԭ�ӣ�
��
��RCN![]() RCOOH
RCOOH
��
�ش��������⣺
(1)F�Ľṹ��ʽ��_________________________________________��
(2)����˵����ȷ����________��
A.������A�Ĺ��������ǻ�
B.������B�ɷ�����ȥ��Ӧ
C.������C�ܷ����ӳɷ�Ӧ
D.������D�ɷ����Ӿ۷�Ӧ
(3)д����˹����������NaOHˮ��Һ��ַ�Ӧ�Ļ�ѧ����ʽ��_________��
(4)д��ͬʱ�������������� D��ͬ���칹��Ľṹ��ʽ��_________��
�������ֻ�ѧ������ͬ����ԭ�ӣ��ں��������������ʡ�
(5)���������ϳ�·�ߣ����һ���ɼ�ȩΪ��ʼԭ���Ʊ���������ĺϳ�·��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������µ���л���ˮ(��CH3COOH)���ɻ�������ԴH2��ԭ����ͼ��ʾ����ȷ���ǣ� ��

A.ͨ���H+ͨ�����ӽ���Ĥ�����ƶ��������Ҳ���ҺpH��С
B.��ԴA��Ϊ����
C.ͨ�������22.4LH2���ɣ���ת��0.2mol����
D.���ԴA�������Ķ��Ե缫�Ϸ����ķ�ӦΪCH3COOH-8e-+2H2O=CO2��+8H+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ����Ҫ�Ļ���ԭ����Ŀǰ�Ƽҵ��Ҫ������������������Ƽ�����ֹ��ա��밴Ҫ��ش����⡣
��1�����������������CaCl2����������д���ù����в���CaCl2�Ļ�ѧ����ʽ��___��
��2��д���������Ƽ�����йط�Ӧ�Ļ�ѧ����ʽ��_____________��___________��
��3��CO2���Ƽҵ����Ҫԭ�����������Ƽ�������������CO2����Դ�кβ�ͬ?________��
��4���������Ƽ���Ƕ���������ĸĽ���������ŵ���___________ (����ĸ���)��
A. �����ԭ��������
B. �����������ɱ�
C. �����˻�����Ⱦ
D. �����˶��豸�ĸ�ʴ
��5����Ʒ�����г�����̼������������ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�������������֪��ȡ��Ʒ������Ϊm1g�����Ⱥ���ʣ���������Ϊm2g������̼�����Ƶ����������ɱ�ʾΪ__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��û��ͨ���ʱ��ȡ����������ʱ��Ƶ�װ��ͼ��ͼ��A��B�ǿɿ��Ƶĵ������С�
(1)�ձ���ʢ�ŵ�Һ����__________����������_________��ˮ����ʢ�ŵ�Һ����_________��
(2)�����������̣�(�������Ѿ�������ʼ)���ռ�����֮ǰ��Ӧ__________________________�����������ȺϺ�Ҫ��ʱ��Ӧ__________________________________���������ռ����ʱ��Ӧ______��
(3)��8.7 g���������뺬�Ȼ���14.6 g��Ũ���Ṳ������������ͬѧ��Ϊ���Ƶ����� 7.1 g����ͬѧ��Ϊ�Ƶ�����������С��7.1 g������Ϊ________(��ס����ҡ�)ͬѧ��ȷ��ԭ����_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����º������������ܱ������е����ʵ���ͨ��CO2��H2����ӦCO2+3H2CH3OH+H2O(g)������������˵���Ѿ��ﵽƽ��״̬���ǣ� ��
A.������CO2������������ٱ仯
B.��CO2��H2ת���ʵı�ֵ���ٱ仯
C.��λʱ���ڶ���3NA��O-H����ͬʱ����3NA��H-H��
D.�����ڻ�������ƽ����Է�������Ϊ34.5���ұ��ֲ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ��������������ó��Ľ�����ȷ����
ѡ�� | ʵ����������� | ���� |
A | ����Һ�еμ�Na2CO3��Һ����Һ���� | ���ӵ�����ǿ��H2CO3������ |
B | ����������������Ƶ��Ҵ���Һ���Ⱥ����������ͨ��������Ȼ�̼��Һ����Һ��ɫ | �����鷢����ȥ��Ӧ |
C |
|
|
D | �� |
|
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�������£���amolN2��bmolH2�Ļ������ͨ��һ���̶��ݻ����ܱ������У��������·�Ӧ��N2(g)��3H2(g)![]() 2NH3(g)
2NH3(g)
������Ӧijʱ��tʱ��n(N2)=13mol��n(NH3)=6mol����a=_____mol��
�ڷ�Ӧ��ƽ��ʱ�������������Ϊ716.8L������£�������NH3�ĺ���(�������)Ϊ25%��ƽ��ʱNH3�����ʵ���_____��
��ԭ���������ƽ��������������ʵ���֮�ȣ�д����������ȡ���ͬ����n(ʼ)��n(ƽ)=_____��
��ԭ��������У�a��b=_____��
�ݴﵽƽ��ʱ��N2��H2��ת����֮�ȣ���(N2)����(H2)=______��ƽ���������У�n(N2)��n(H2)��n(NH3)=_____��
��2��CH3OHȼ�ϵ����Ŀǰ������ɹ���ȼ�ϵ��֮һ������ȼ�ϵ���ɼ״�������(����)��KOH(�������Һ)���ɡ����и�����ӦʽΪCH3OH��8OH--6e-=CO32-��6H2O��������˵����ȷ����______(�����)��
�ٵ�طŵ�ʱͨ������ĵ缫Ϊ����
�ڵ�طŵ�ʱ���������Һ�ļ�������
�۵�طŵ�ʱÿ����6.4gCH3OHת��1.2mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ĿҪ���û�ѧ���Իش����⡣
��I����ȥ���������ڵ�����ͨ�����õ�ʵ�鷽����ʲô���������ں����ϡ�
(1)CH3CH2OH(H2O)_____________________________________________________��
(2)![]() (NaCl)____________________________________________��
(NaCl)____________________________________________��
(3) ![]() (Br2)___________________________________________________��
(Br2)___________________________________________________��
��II��ij�л����ʵ��ʽΪC2H6O���������Dzⶨ����Է������������ⶨ�õ���ͼ1��ʾ������ͼ������ú˴Ź����Ǵ������л���õ���ͼ2��ʾ�ĺ˴Ź�������ͼ��

�Իش��������⣺
(1)���л����������Է�������Ϊ________��
(2)��д�����л�������Ľṹ��ʽ________��
��III���л���E(C3H3Cl3)��һ�ֲ�ǰ���ݼ���ǰ�壬��ϳ�·�����¡�

��֪D�ڷ�Ӧ���������ɵ�E����ṹֻ��һ�ֿ��ܣ�E��������3�ֲ�ͬ���͵���(�����ǿռ��칹)���Իش��������⣺
(1)��������е���Ϣ�Ʋ���A�Ľṹ��ʽΪ______________����A��ͬϵ���У���Է���������С���������Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ____________________________��
(2)д�����з�Ӧ�����ͣ���Ӧ����____________����Ӧ����________��
(3)��������е���Ϣ�Ʋ��л���D��������______________��
(4)��д����Ӧ�۵Ļ�ѧ����ʽ��____________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com