
ЁОЬтФПЁПаТаЭОЛЫЎМСИпЬњЫсМи(K2FeO4)ЮЊАЕзЯЩЋЙЬЬхЃЌПЩШмгкЫЎЃЌдкжаадЛђЫсадШмвКжаж№НЅЗжНтЃЌдкМюадШмвКжаЮШЖЈЁЃЙЄвЕЩЯжЦБИK2FeO4ЕФГЃгУЗНЗЈгаСНжжЁЃ
ЗНЗЈЂёЃКДЮТШЫсбЮбѕЛЏЗЈЁЃЙЄвеСїГЬШчЭМЫљЪОЁЃ
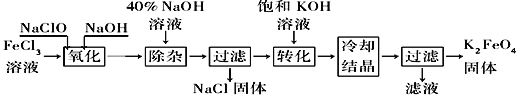
ЃЈ1ЃЉЭъГЩЁАбѕЛЏЁБЙ§ГЬжаЗДгІЕФЛЏбЇЗНГЬЪНЃК______FeCl3ЃЋ______NaOHЃЋ______NaClO==______Na2FeO4ЃЋ______![]() ЃЋ______
ЃЋ______![]() ЁЃ____________ЦфжабѕЛЏМСЪЧ________(ЬюЛЏбЇЪН)ЁЃ
ЁЃ____________ЦфжабѕЛЏМСЪЧ________(ЬюЛЏбЇЪН)ЁЃ
ЃЈ2ЃЉЁАзЊЛЏЁБЙ§ГЬжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ____________________________________ЁЃ
ЃЈ3ЃЉЩЯЪіЙЄвеЕУЕНЕФИпЬњЫсМиГЃКЌгадгжЪЃЌПЩгУжиНсОЇЗЈЬсДПЃЌВйзїЪЧНЋДжВњЦЗгУ________________ШмНтЃЌШЛКѓ________________ЁЃ
ЗНЗЈЂђЃКЕчНтЗЈЁЃвдЬњЮЊбєМЋЕчНтЧтбѕЛЏФЦШмвКЃЌШЛКѓдкбєМЋвКжаМгШыKOHЁЃ
ЃЈ4ЃЉЕчНтЪБбєМЋЗЂЩњЗДгІЩњГЩFeO42ЃЃЌИУЕчМЋЗДгІЗНГЬЪНЮЊ________________________________ЁЃ
ЁОД№АИЁП2ЁЁ10ЁЁ3ЁЁ2ЁЁ9ЁЁNaClЁЁ5ЁЁH2ONaClONa2FeO4ЃЋ2KOH===K2FeO4ЃЋ2NaOHЯЁKOHШмвКМгШыБЅКЭKOHШмвКЃЌРфШДНсОЇFeЃЋ8OHЃЃ6eЃ=FeO42-ЃЋ4H2O
ЁОНтЮіЁП
ЃЈ1ЃЉFeЕФЛЏКЯМлгЩЃЋ3МлЁњЃЋ6МлЃЌЛЏКЯМлЩ§Ип3МлЃЌClЕФЛЏКЯМлгЩЃЋ1МлЁњЃ1МлЃЌЛЏКЯМлНЕЕЭ2МлЃЌзюаЁЙЋБЖЪ§ЮЊ6ЃЌШЛКѓИљОндзгЪиКуХфЦНЦфЫћМДПЩЃЌМДЗДгІЗНГЬЪНЮЊ2FeCl3ЃЋ10NaClЃЋ3NaClO=2Na2FeO4ЃЋ9NaClЃЋ5H2OЃЌЦфжаNaClOЮЊбѕЛЏМСЃЛЃЈ2ЃЉИљОнСїГЬЃЌЙ§ТЫКѓТЫвКжаКЌгаNa2FeO4ЃЌРћгУK2FeO4ЕФШмНтЖШаЁгкNa2FeO4ЃЌвђДЫЗЂЩњЕФЗДгІЗНГЬЪНЮЊNa2FeO4ЃЋ2KOH=K2FeO4ЃЋ2NaOHЃЛЃЈ3ЃЉвђЮЊИпЬњЫсМидкжаадЛђЫсадШмвКжаж№НЅЗжНтЃЌдкМюадШмвКжаЮШЖЈДцдкЃЌвђДЫЯШгУЯЁKOHШмвКШмНтЃЌШЛКѓМгШыБЅКЭKOHШмвКЃЌРфШДНсОЇЃЛЃЈ4ЃЉЕчНтжЪЮЊKOHЃЌЬњзїбєМЋЃЌвђДЫбєМЋЕчМЋЗДгІЪНЮЊFeЃЋ8OHЃЃ6eЃ=FeO42ЃЃЋ4H2OЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЃЈ1ЃЉЛљЬЌGaдзгжаФмСПзюИпЕФФмВуЗћКХ________ га______ жжВЛЭЌФмСПЕФЕчзг,GaдЊЫигыЭЌжмЦкЯрСкдЊЫиZnЁЂGeЯрБШЃЌЕквЛЕчРыФмДгДѓЕНаЁЕФЫГађ__________,ЃЈгУдЊЫиЗћКХБэЪОЃЉН№ЪєZnОЇЬхВЩгУСљЗНзюУмЖбЛ§ЃЌЦфУмжУВуЖбЛ§ЗНЪНЮЊ__________ ЃЈABABABЛђABCABCЃЉЧјЗжОЇЬхКЭЗЧОЇЬхзюПЩППЕФПЦбЇЗНЗЈЪЧ________________ЁЃ
ЃЈ2ЃЉСзгаШ§жжКЌбѕЫс H3PO2 ЁЂ H3PO3 ЁЂ H3PO4 ЦфжаСздзгОљвдsp3дгЛЏгыЯрСкдзгаЮГЩЫФИіІС МќЃЌдђЂйH3PO3ЕФНсЙЙЪНЪЧ__________ЃЛЂкаДГіH3PO2 гызуСПЧПбѕЛЏФЦШмвКЗДгІЕФЛЏбЇЗНГЬЪН_________ЃЛЂлШ§жжЫсЕФЧПШѕЫГађЮЊH3PO2 <H3PO3 < H3PO4ЃЌЦфдвђЪЧ___________ЃЛЂмHNO3 ЁЂ HNO2 жааФдзгЕФдгЛЏЗНЪНЗжБ№ЮЊ__________ЁЃ
ЃЈ3ЃЉН№ЪєЭЭЖШыАБЫЎЛђЙ§бѕЛЏЧтШмвКжаОљЮоУїЯдЯжЯѓЃЌЕЋЭЖШыАБЫЎКЭЙ§бѕЛЏЧтЕФЛьКЯШмвКжаЃЌдђЭЦЌШмНтЃЌШмвКГЪЩюРЖЩЋЁЃЂйаДГіИУЗДгІЕФРызгЗДгІЗНГЬЪН____________________________ ЃЛЂквбжЊNF3гыNH3ЕФПеМфЙЙаЭЖМЪЧШ§НЧзЖаЮЃЌЕЋNF3ВЛвзгыCu2+аЮГЩХфРызгЃЌдвђЪЧ_______________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПеЦЮевЧЦїУћГЦЁЂзщзАМАЪЙгУЗНЗЈЪЧжабЇЛЏбЇЪЕбщЕФЛљДЁЃЌШчЭМЮЊеєСѓЪЕбщзАжУЁЃ
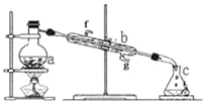
ЃЈ1ЃЉаДГіЯТСавЧЦїЕФУћГЦЃКaЃЎ__bЃЎ__ЁЃ
ЃЈ2ЃЉЪЕбщЙ§ГЬжаЃЌашвЊЭЈРфЫЎЃЌЭМжаЕФНјЫЎЗНЯђЪЧ__НјЃЈЬюЭМжазжФИЃЉЁЃ
ЃЈ3ЃЉШєРћгУзАжУЗжРыЫФТШЛЏЬМКЭОЦОЋЕФЛьКЯЮяЃЌЛЙШБЩйЕФгУЦЗЪЧ__ЁЃ
ЃЈ4ЃЉШєгУзАжУжЦеєСѓЫЎЃЌЪЕбщЪБaжаГ§МгШыЩйСПздРДЫЎЭтЃЌЛЙашМгШыЩйСП__ЃЌЦфзїгУЪЧЗРжЙБЉЗаЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПДПОЛЮяXЁЂYЁЂZзЊЛЏЙиЯЕШчЭМЫљЪОЃЌЯТСаХаЖЯе§ШЗЕФЪЧ
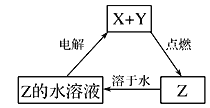
A. XПЩФмЪЧН№ЪєЭ B. YВЛПЩФмЪЧЧтЦј
C. ZПЩФмЪЧШ§бѕЛЏСђ D. ZПЩФмЪЧТШЛЏФЦ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЮяжЪЪєгкДПОЛЮяЕФЪЧ( )
ЂйАБЫЎЂкжиЫЎЂлУїЗЏЁЁЂмДПМюЁЁЂнЬьШЛЦјЁЁЂобѕЦјЁЁЂпЦЏАзЗлЁЁЂрТСШШМС
A. ЂйЂмЂнЂпB. ЂкЂлЂмЂоC. ЂмЂпЂрD. ЂлЂнЂоЂр
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊЃКвЛИіЬМдзгЩЯСЌЖрИієЧЛљЪБВЛЮШЖЈЃК
ЗМЯуЬўAгаШчЭМЕФзЊЛЏЙиЯЕЃК
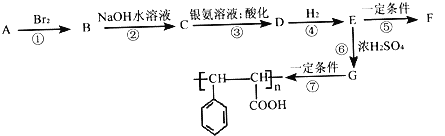
ЃЈ1ЃЉвбжЊ1molAФмгы2molBr2МгГЩЃЌаДГіAЕФНсЙЙМђЪН___________________ЃЛаДГіBЕФЗжзгЪН___________________ЁЃ
ЃЈ2ЃЉDжаКЌбѕЙйФмЭХЕФУћГЦЮЊ______________ЃЌжИГіЗДгІЂоЕФЗДгІРраЭЃК___________________ЁЃ
ЃЈ3ЃЉаДГіCгывјАБШмвКЗДгІЕФЛЏбЇЗНГЬЪНЃК_________________________________ЁЃ
ЃЈ4ЃЉEгаЖржжЭЌЗжвьЙЙЬхЃЌЗћКХЯТСаЬѕМўЕФЙВга__________жжЁЃ
ЂйЪєгкЗМЯуЛЏКЯЮяЃЛ ЂкФмЪЙFeCl3ШмвКЗЂЩњЯдЩЋЗДгІЃЛ
ЂлКЌгаѕЅЕФНсЙЙЃЛ ЂмБНЛЗЩЯгаСНИіШЁДњЛљЁЃ
ЦфжаКЫДХЙВеёЧтЦзжага6зщЮќЪеЗхЃЌЦфУцЛ§жЎБШЮЊ1ЁУ2ЁУ2ЁУ2ЁУ2ЁУ1ЕФгаЛњЮяНсЙЙМђЪНЮЊ___________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЙЄвЕЩЯЭЈГЃгУЙЄвЕОЦОЋКЭЩњЪЏЛвЛьКЯеєСѓЗЈжЦШЁЮоЫЎввДМЁЃШчЭМЪЧЪЕбщЪвжаФЃФтЙЄвЕдРэжЦШЁЮоЫЎввДМЕФзАжУЁЃ
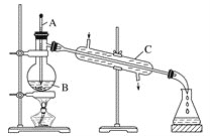
ЛиД№ЯТСаЮЪЬтЃК
(1)жИГіЭМжаЕФШ§ДІДэЮѓЃК________________ЁЂ________________ЁЂ________________ЁЃ
(2)дкГ§ШЅЙЄвЕОЦОЋжаЕФЫЎЪБЃЌЯТСазіЗЈВЛПЩШЁЕФЪЧЃЈ____ЃЉ
AЃЎМгШыЙ§СПЕФЩњЪЏЛвКѓЙ§ТЫ
BЃЎМгШыЙ§СПЕФЩњЪЏЛвКѓеєСѓ
CЃЎМгШыЙ§СПЕФЩњЪЏЛвКѓЗжвК
(3)гЩ(2)ПЩжЊГ§ШЅЙЄвЕОЦОЋжаЕФЫЎЕФдРэЪЧ(гУЛЏбЇЗНГЬЪНБэЪО)______________________ЁЃ
(4)вЧЦїAЕФУћГЦЪЧ____________ЃЌвЧЦїBЕФУћГЦЪЧ____________ЃЌвЧЦїCЕФУћГЦЪЧ____________ЁЃ
(5)дкеєСѓВйзїжаЃЌвЧЦїBжаМгШыЗаЪЏ(ЛђЫщДЩЦЌ)ЕФзїгУЪЧ______________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТБэЪЧдЊЫижмЦкБэЕФвЛВПЗжЃЌеыЖдБэжаЕФЂйЁЋЂтжадЊЫиЃЌгУдЊЫиЗћКХЛђЛЏбЇЪНЬюПеЛиД№вдЯТЮЪЬтЃК
IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 | |
Жў | Ђй | Ђк | ||||||
Ш§ | Ђл | Ђм | Ђн | Ђо | Ђп | Ђр | ||
ЫФ | Ђс | Ђт |
(1)дкетаЉдЊЫижаЃЌзювзЪЇЕчзгЕФдЊЫиЪЧ________ЃЌЗЧН№ЪєадзюЧПЕФдЊЫиЪЧ______ЃЛ
(2)ЛЏбЇаджЪзюВЛЛюЦУЕФдЊЫиЪЧ_____ЃЌЦфдзгЕФдзгНсЙЙЪОвтЭМЮЊ________ЃЛ
(3)дЊЫиЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяжаЫсадзюЧПЕФЪЧ____ЃЌМюадзюЧПЕФЪЧ___ЃЌГЪСНадЕФЧтбѕЛЏЮяЪЧ_______________ЃЛ(ЬюаДЛЏбЇЪН)
(4)дкЂлЁЋЂпдЊЫижаЃЌМђЕЅРызгАыОЖзюаЁЕФЪЧ_________ЃЛ
(5дкЂпгыЂтЕФЕЅжЪжаЃЌбѕЛЏадНЯЧПЕФЪЧ_______________ЃЌгУЗДгІЛЏбЇЗНГЬЪНжЄУїЃК_____________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЪжЛњаОЦЌЪЧвЛжждкАыЕМЬхВФСЯЩЯМЏКЯЖржжЕчзгдЊЦїМўЕФЕчТЗФЃПщЁЃЯТСаПЩгУзїАыЕМЬхВФСЯЕФЪЧЃЈ ЃЉ
A. ТС B. ЖўбѕЛЏЙш C. Ьњ D. Йш
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com