
����Ŀ��
���������������ճ�����������Ӧ�ù㷺�IJ��ϡ���ش��������⣺
��l����̬��ԭ�ӵļ۵��ӹ������ʽΪ__________��
��2����Ԫ�س�����������Fe2+��Fe3+���ȶ���Fe2+_______Fe2+����������������С����)��ԭ����________________��
��3�������������ܴ�����ƽ���NH4ClO4�ķֽ⣬NH4+�ĽṹʽΪ______�������λ�������ռ乹��Ϊ_________�����е�ԭ�ӵ��ӻ���ʽΪ_______����ClO4-��Ϊ�ȵ�����ķ��ӻ�������__________����д���֣���
��4������������ԭ�Ӳ���________�ѻ���������Ŀռ�������Ϊ______���ú�����ʽ�ӱ�ʾ����
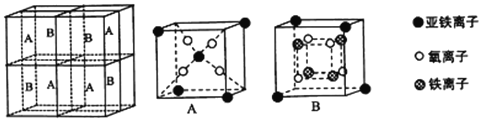
��5��ij�����������������ᄃ����ͼ��ʾ������A��B������ɡ����Ȩ������Fe2+��Fe3+��O2-�ĸ�����Ϊ_______������������ȣ�����֪�þ�����ܶ�Ϊdg/cm3�������ӵ�������ֵΪNA����Ʒ������a Ϊ_______nm���ú�d ��NA�Ĵ���ʽ��ʾ����
���𰸡� 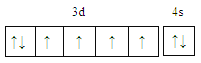 С�� Fe2+�ļ۵����Ų�ʽΪ3d6��Fe3+�ļ۵����Ų�ʽΪ3d5��Fe3+ ��3d�ܼ�Ϊ����״̬���ȶ���
С�� Fe2+�ļ۵����Ų�ʽΪ3d6��Fe3+�ļ۵����Ų�ʽΪ3d5��Fe3+ ��3d�ܼ�Ϊ����״̬���ȶ��� ![]() ���������� sp3�ӻ� CCl4��PO43- �����������𰸾��ɣ� ��������
���������� sp3�ӻ� CCl4��PO43- �����������𰸾��ɣ� �������� ![]() 1:2:4
1:2:4 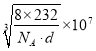
�����������������������Ҫ����ԭ�ӽṹ�뾧��ṹ��
��l����̬��ԭ�ӵļ۵��ӹ������ʽΪ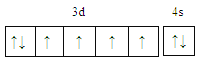 ��
��
��2����Ԫ�س�����������Fe2+��Fe3+���ȶ���Fe2+Fe2+����������������С����)��ԭ����Fe2+�ļ۵����Ų�ʽΪ3d6��Fe3+�ļ۵����Ų�ʽΪ3d5��Fe3+ ��3d�ܼ�Ϊ����״̬���ȶ�����
��3�������������ܴ�����ƽ���NH4ClO4�ķֽ⣬NH4+�ĽṹʽΪ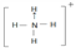 ���ռ乹��Ϊ���������Σ����е�ԭ�ӵļ۲���Ӷ�Ϊ4���ӻ���ʽΪsp3�ӻ���ClO4-��5ԭ�ӡ�32�۵��ӵ����ӣ���ClO4-��Ϊ�ȵ�����ķ��ӻ�������CCl4��PO43-��
���ռ乹��Ϊ���������Σ����е�ԭ�ӵļ۲���Ӷ�Ϊ4���ӻ���ʽΪsp3�ӻ���ClO4-��5ԭ�ӡ�32�۵��ӵ����ӣ���ClO4-��Ϊ�ȵ�����ķ��ӻ�������CCl4��PO43-��
��4������������ԭ�Ӳ������������ѻ���������ľ�������2����ԭ�ӣ��辧���߳�Ϊa������ԭ�Ӱ뾶Ϊr��
����Խ��߳�![]() a����Խ��߳�
a����Խ��߳�![]() a=4r����r=
a=4r����r=![]() ���ռ�������=
���ռ�������=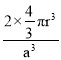 =
= ![]() ��
��
��5��A����1.5���������ӡ�4�������ӣ�B����0.5���������ӡ�4�������ӡ�4�������ӣ������������Fe2+��Fe3+��O2-�ĸ�����Ϊ1:2:4��
��������Fe2+��Fe3+��O2-�ĸ����ֱ���Ϊ4��8��16�����ǵ��������֮����8��232������m=��V�ɵ�8��232 g=d��g/cm3a3��NA��a =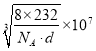 nm��
nm��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б仯�е���ֻ���ֳ����Ե���(����)
A. Ũ���������ǻ�� B. Ũ������ͭ��Ӧ
C. ϡ������BaCl2��Һ��� D. ������Na2CO3��Һ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й�����,�����й����ݻش���������:
����
����ʽ:HCl
��Է�������:36.5
�ܶ�:1.19g/cm3
��������:36.5%
��1����Ũ������HCl�����ʵ���Ũ��Ϊ_______mol/L.
��2��ȡ����������ĸ�������Һʱ,����������������ȡ������ٶ��仯��_________��
A����Һ��H+�����ʵ���Ũ��B����Һ��HCl������
C����Һ��H+����ĿD����Һ���ܶ�
��3��������1L 1mol/L��ϡ����,��ʹ��Ũ������1��,��ȡ�Ĵ�ʩ���������____________��
A��ͨ������HCl����22.4L
B������Һ����Ũ����0.5L
C����ԭ��Һ����5mol/L����0.6L,��ϡ����2L
D����ԭ��Һ����1L 3mol/L�����Ͼ���.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���0.100mol��L-1��NaOH��Һ�ֱ�ζ���Ϊ20.00mL0.100mol��L-1��HCl��Һ�ʹ�����Һ���ζ�������ͼ��ʾ������˵����ȷ����

A. I��ʾ���ǵζ����������
B. pH =7ʱ���ζ��������ĵ�V(NaOH)��20.00mL
C. V(NaOH)= 20.00mLʱ��������Һ��c(Cl-)��c(CH3COO-)
D. V(NaOH)=10.00mLʱ��������c(Na+)��c(CH3COO-)��c(H+)��c(OH-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������NaOH��������1.0mol��L��1 ��NaOH��Һ 240mL
��1��������Һʱ,һ����Է�Ϊ���¼�������:
�ٳ��� �ڼ��� ���ܽ� �ܵ�תҡ�� ��ת�Ƣ�ϴ�� �߶��� ����ȴ
��ʵ������õ�����������ƽ��ҩ�ס����������ձ�����ͷ�ιܡ�����_________.
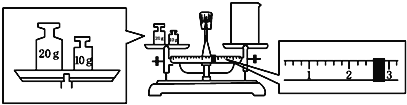
��2��ijͬѧ������һ������NaOH����,������������ƽ�����ձ�������,��ƽƽ����״̬��ͼ.�ձ���ʵ������Ϊ__________g,Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ�__________gNaOH.
��3��ʹ������ƿǰ������е�һ��������_________________________________.
��4�������ƹ�����,��������������ȷ��,���в���������Ũ��ƫ�ߵ���________________.
��û��ϴ���ձ��Ͳ�����
��ת����Һʱ������������������ƿ����
������ƿ������,������������ˮ
�ܶ���ʱ���ӿ̶���
��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
���ݺ�����ƿ������ҡ��,���ú�,Һ����ڿ̶���,�ټ�ˮ���̶���.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л���������ͬ���칹����Ŀ�����ǣ� ��
A.C8H10(������)B.C5H10O2(��)C.C4H8Cl2D.C4H8O2(��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ��Ӧ�У�����������ԭ��Ӧ����
A. CaCO3 + 2HCl �� CaCl2 + H2O + CO2��
B. NaCl+AgNO3��AgCl��+NaNO3
C. CaCO3 ![]() CaO+CO2��
CaO+CO2��
D. 2Na + 2H2O ��2NaOH + H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����Ԫ��R����������ϼ���������ϼ۴�����Ϊ4��������������ȷ����
A. R����̬�⻯��ΪH2R B. R������������ˮ����ΪH2RO4
C. Rһ���ǵ�IVA��Ԫ�� D. R�����������ΪRO3
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com