
������(H3PO2)��һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԡ��ش��������⣺
(1)H3PO2��һ����ǿ�ᣬд������뷽��ʽ��_____________________________
________________________________________________________________________��
(2)H3PO2��NaH2PO2���ɽ���Һ�е�Ag����ԭΪ�����Ӷ������ڻ�ѧ������
��H3PO2�У�PԪ�صĻ��ϼ�Ϊ________��
������H3PO2���л�ѧ������Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ4��1������������Ϊ________(�ѧʽ)��
��NaH2PO2Ϊ________(����Ρ�����ʽ�Ρ�)������Һ��________(������ԡ������ԡ��������ԡ�)��
(3)H3PO2�Ĺ�ҵ�Ʒ��ǣ�������(P4)��Ba(OH)2��Һ��Ӧ����PH3�����Ba(H2PO2)2����������H2SO4��Ӧ��д��������Ba(OH)2��Һ��Ӧ�Ļ�ѧ����ʽ��____________________________________________��
(4)H3PO2Ҳ���õ��������Ʊ��������ҵ�������������ԭ����ͼ��ʾ(��Ĥ����Ĥ�ֱ�ֻ���������ӡ�������ͨ��)��
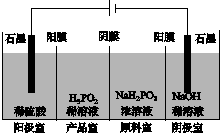
��д�������ĵ缫��Ӧʽ��_________________________________________��
�ڷ�����Ʒ�ҿɵõ�H3PO2��ԭ��_____________________________________
________________________________________________________________________��
�����ڲ��á����ҵ����������Ʊ�H3PO2���������ҵ����������������ҵ�ϡ������H3PO2ϡ��Һ���棬����ȥ���������Ʒ��֮�����Ĥ���Ӷ��ϲ������������Ʒ�ҡ���ȱ���Dz�Ʒ�л���________���ʣ������ʲ�����ԭ����________________________________��
(1)H3PO2 H2PO
H2PO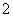 ��H����(2)�٣�1����H3PO4�������Ρ�������
��H����(2)�٣�1����H3PO4�������Ρ�������
(3)2P4��3Ba(OH)2��6H2O��3Ba(H2PO2)2��2PH3��
(4)��2H2O��4e��===O2����4H��
�������ҵ�H��������Ĥ��ɢ����Ʒ�ң�ԭ���ҵ�H2PO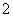 ������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2����PO
������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2����PO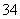 ��H2PO
��H2PO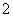 ��H3PO2������
��H3PO2������
[����] (1)H3PO2ΪһԪ���ᣬ����뷽��ʽΪH3PO2 H����H2PO
H����H2PO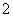 ��(2)�ɻ��ϼ۴�����Ϊ0��ȷ��PΪ��1�ۣ��ڸ���������Ϣд����ѧ����ʽΪ4Ag����H3PO2��2H2O===4Ag��H3PO4��4H��������������ΪH3PO4����NaH2PO2Ϊǿ�������Σ���Һ�������ԡ�(3)����������Ϣ�ͷ�Ӧǰ��Ԫ�ػ��ϼ۱仯д����ѧ����ʽΪ2P4��3Ba(OH)2��6H2O===2PH3����3Ba(H2PO2)2��(4)��������ˮ�������OH���ŵ磬�䷴ӦʽΪ2H2O��4e��===O2����4H�������������е�H��������Ĥ�����Ʒ�ң�ԭ���ҵ�H2PO
��(2)�ɻ��ϼ۴�����Ϊ0��ȷ��PΪ��1�ۣ��ڸ���������Ϣд����ѧ����ʽΪ4Ag����H3PO2��2H2O===4Ag��H3PO4��4H��������������ΪH3PO4����NaH2PO2Ϊǿ�������Σ���Һ�������ԡ�(3)����������Ϣ�ͷ�Ӧǰ��Ԫ�ػ��ϼ۱仯д����ѧ����ʽΪ2P4��3Ba(OH)2��6H2O===2PH3����3Ba(H2PO2)2��(4)��������ˮ�������OH���ŵ磬�䷴ӦʽΪ2H2O��4e��===O2����4H�������������е�H��������Ĥ�����Ʒ�ң�ԭ���ҵ�H2PO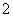 ������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2�����������ڿ����в���H2PO
������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2�����������ڿ����в���H2PO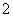 ��H3PO2ʧ���ӷ���������Ӧ�������������л���PO
��H3PO2ʧ���ӷ���������Ӧ�������������л���PO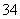 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0. 03molCl2����ͨ�뺬0.02mol H2SO3��0.02molHBr�Ļ����Һ�У��ڴ˹�������Һ��c(H+)��Cl2�����Ĺ�ϵʾ��ͼ��(��Һ�������Ϊ����)
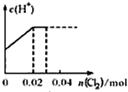
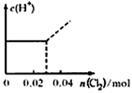
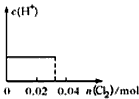
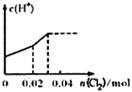
A B C D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����  ���ף����ױȰ����ȶ����ṹ��ʯī���ƣ�����������ȷ�ģ� ��
���ף����ױȰ����ȶ����ṹ��ʯī���ƣ�����������ȷ�ģ� ��
A.���������Ϊͬ���칹�� B. ����ת��Ϊ�����Ƿ��ȷ�Ӧ
C.����ת��Ϊ���� ��������ԭ��Ӧ D. ���Ͱ������ܵ���
��������ԭ��Ӧ D. ���Ͱ������ܵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�ͬ���칹����Ŀ�������У�����ȷ����( )
A.�ױ������ϵ�һ����ԭ�ӱ���3��̼ԭ�ӵ����ȡ�������ò�����6��
B.����ʽ����C5H11Cl �Ļ�������6��
C.��֪���ȱ���3��ͬ���칹�壬�����ȱ���ͬ���칹�����ĿΪ3��
D.�ƵĽṹ��ʽΪ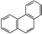 ���������ᷴӦ��������5��һ����ȡ����
���������ᷴӦ��������5��һ����ȡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£�������Һ������Ũ�ȹ�ϵʽ��ȷ����(����)
A��pH��5��H2S��Һ�У�c(H��)��c(HS��)��1��10��5 mol��L��1
B��pH��a�İ�ˮ��Һ��ϡ��10������pH��b����a��b��1
C��pH��2��H2C2O4��Һ��pH��12��NaOH��Һ���������ϣ�c(Na��)��c(H��)��c(OH��)��c(HC2O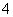 )
)
D��pH��ͬ�Ģ�CH3COONa����NaHCO3����NaClO������Һ��c(Na��)���٣��ڣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���0.10 mol��L��1 NaHCO3��Һ��˵����ȷ����(����)
A�����ʵĵ��뷽��ʽΪNaHCO3===Na����H����CO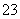
B��25 ��ʱ����ˮϡ�ͺ�n(H��)��n(OH��)�ij˻����
C������Ũ�ȹ�ϵ��c(Na��)��c(H��)��c(OH��)��c(HCO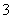 )��c(CO
)��c(CO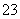 )
)
D. �¶����ߣ�c(HCO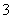 )����
)����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�������Һ������Ũ�ȹ�ϵ��ȷ����(����)
A��Na2S��Һ��c(Na��)>c(HS��)>c(OH��)>c(H2S)
B��Na2C2O4��Һ��c(OH��)��c(H��)��c(HC2O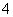 )��2c(H2C2O4)
)��2c(H2C2O4)
C��Na2CO3��Һ��c(Na��)��c(H��)��2c(CO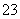 )��c(OH��)
)��c(OH��)
D��CH3COONa��CaCl2�����Һ��c(Na��)��c(Ca2��)��c(CH3COO��)��c(CH3COOH)��2c(Cl��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ⱦ�����Чȥ������Դ�ij�������ǻ�ѧ�츣�������Ҫ�о����⡣ij�о�С���������̿�(��Ҫ�ɷ�ΪMnO2��������������������ͭ�����Ƚ���������)���������ͨ�����¼����̼��ѳ�ȼúβ���е�SO2�����Ƶõ�ز���MnO2(��Ӧ������ʡ��)��
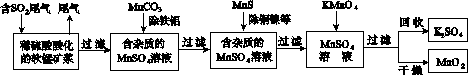
��ش��������⣺
(1)������������ʵ����____(ѡ��������ĸ���)��
A����������ۺ�����
B����ɫ��Ⱦ�ļ���
C������ļ���
(2)��MnCO3�ܳ�ȥ��Һ�е�Al3����Fe3������ԭ����________________________________��
(3)��֪��25 �桢101 kPaʱ��
Mn(s)��O2(g)===MnO2(s)����H����520 kJ/mol
S(s)��O2(g)===SO2(g)����H����297 kJ/mol
Mn(s)��S(s)��2O2(g)===MnSO4(s)��
��H����1065 kJ/mol
SO2��MnO2��Ӧ������ˮMnSO4���Ȼ�����ʽ��____________________________________��
(4)MnO2�����������������ϡ��ö��Ե缫���MnSO4��Һ���Ƶ�MnO2���������ĵ缫��Ӧʽ��________________________________��
(5)MnO2�Ǽ���п�̵�ص��������ϡ�����п�̵�طŵ�ʱ�������ĵ缫��Ӧʽ��________________��
(6)�����ѳ���SO2ֻ�����̿��е�MnO2��Ӧ������ͼʾ���̣���a m3(��״��)��SO2���������Ϊb%��β��ͨ�����SO2���ѳ���Ϊ89.6%�����յõ�MnO2������Ϊc kg�����ȥ��������ͭ����������ʱ�����������Ԫ���൱��MnO2________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б�ʾ��Һ�з�����Ӧ�Ļ�ѧ����ʽ�������( )
A.2Al+2NaOH+2H2O====2NaAlO2+3H2��
B.2KMnO4+HCOOK+KOH====2K2MnO4+CO2��+H2O
C.MnO2+4HCl(Ũ)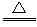 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
D.K2Cr2O7+6FeSO4+7H2SO4====Cr2(SO4)3+3Fe2(SO4)3+K2SO4+7H2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com