



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����״���£�32gO2ռ�е����ԼΪ22.4L |
| B��������Ħ��������2g |
| C���ڱ�״���£�22.4Lˮ������Ϊ18g |
| D��1molH2O��������18g/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ر�� | Ԫ��������Ϣ |
| A | �䵥�����ܶ���С������ |
| B | �����Ӵ�������λ����ɣ������ǿ�������Ҫ�ɷ�֮һ |
| C | ����������B������������ͬ�ĵ��Ӳ�ṹ�� ����B�����γ��������ӻ����� |
| D | ����������������ﶼ�����ԣ���Cͬ���� |
| E | ��Cͬ���ڣ�ԭ�Ӱ뾶�ڸ�������С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ܢ� | B���٢ۢ� |
| C���٢ۢݢ� | D���ۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
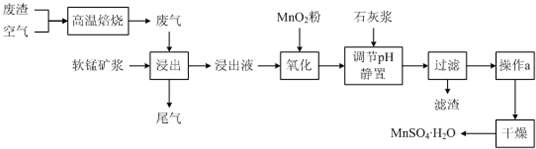
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 7.6 | 9.7 |
| Fe3+ | 2.7 | 3.7 |
| Al3+ | 3.8 | 4.7 |
| Mn2+ | 8.3 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
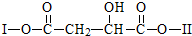 ������I��IIΪδ֪���ֵĽṹ��Ϊ�Ʋ�X�ķ��ӽṹ��������ͼ��ת����
������I��IIΪδ֪���ֵĽṹ��Ϊ�Ʋ�X�ķ��ӽṹ��������ͼ��ת����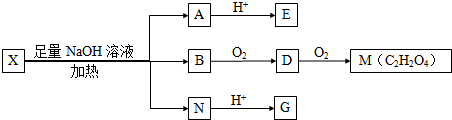
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
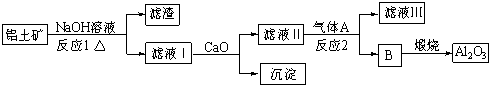
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
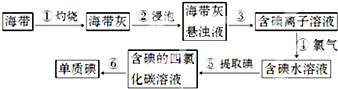
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com