
����Ŀ���밴������Ҫ��ش����⣺
��1������Ȼ�����Һ�Ļ�ѧ����ʽ��__________________________________��
��2������ˮ������ӷ���ʽ��________________________________��
��3��0.1 mol/L ��̼������Һ������Ũ���ɴ�С˳��Ϊ��__________________________��
��4��Ũ�� Al2(SO4)3 ��Һ��Ũ��С�մ�(NaHCO3)��Һ��Ͽ���������������ӷ�Ӧ����ʽ��ʾ����ԭ��__________________________��
��5����25���� pH=12 �� Ba(OH)2 ��ҺaL�� pH=1��HCl��ҺbL ��ϣ������û��ҺΪ���ԣ��� a��b= __________________________��(��Һ����仯���Բ���)��
��6��pH=3 �� NH4Cl ��Һ����ˮ������� c(H+)= __________________________��
��7������ʱ��Fe(OH)3 ���ܶȻ����� Ksp��1��10��38��Ҫʹ��Һ�е� Fe3��������ȫ(������ c(Fe3��)<10��5 mol��L��1)���� ��Һ�� pH Ӧ����____________________________��
���𰸡�2NaCl��2H2O ![]() Cl2����2NaOH��H2�� S2-+H2O
Cl2����2NaOH��H2�� S2-+H2O![]() HS-+OH- c(Na+) >c(CO32-) >c(OH-) >c(HCO3-) >c(H+) Al3++3HCO3--=Al(OH)3��+3CO2�� 10:1 1��10-3mol/L 3
HS-+OH- c(Na+) >c(CO32-) >c(OH-) >c(HCO3-) >c(H+) Al3++3HCO3--=Al(OH)3��+3CO2�� 10:1 1��10-3mol/L 3
��������
��1���Ȼ�����Һ���������������ơ�������������
��2��S2-ˮ�⣬����HS-��OH-��
��3��̼������Һ�д���Na+��CO32-��H+��OH-������ǿ�������Σ�ˮ��Һ�ʼ��ԣ�����CO32-ˮ������HCO3-��
��4��Al2(SO4)3 ��Һ��С�մ��ϣ�Al3+��HCO3-����˫ˮ�ⷴӦ������CO2��Al(OH)3��
��5�����ҺΪ���ԣ���n(H+)=n(OH-)��
��6��NH4Cl ��ҺΪ����Һ����Һ�е�H+ȫ������ˮ���룻
��7��Ksp��c(Fe3+)c3(OH-)������c(Fe3��)<10��5 mol��L��1�����c (OH-)����һ������c (H+)��ȷ��pH�Ĵ�С��
��1���Ȼ�����Һ���������������ơ���������������Ӧ����ʽΪ��2NaCl��2H2O ![]() Cl2����2NaOH��H2����
Cl2����2NaOH��H2����
��2��S2-ˮ�⣬����HS-��OH-��ˮ�ⷽ��ʽΪ��S2-+H2O![]() HS-+OH-��
HS-+OH-��
��3��̼������Һ�д���Na+��CO32-��H+��OH-������ǿ�������Σ�ˮ��Һ�ʼ��ԣ�����CO32-ˮ������HCO3-����˸����ӵ�Ũ�ȹ�ϵΪ��c(Na+) >c(CO32-) >c(OH-) >c(HCO3-) >c(H+)��
��4��Al2(SO4)3 ��Һ��С�մ��ϣ�Al3+��HCO3-����˫ˮ�ⷴӦ������CO2��Al(OH)3������ʽΪ��Al3++3HCO3--=Al(OH)3��+3CO2����
��5�����ҺΪ���ԣ���n(H+)=n(OH-), pH=12 �� Ba(OH)2 ��ҺaL��n(OH-)=0.01a��pH=1��HCl��ҺbL��n(H+)=0.1b��0.01a=0.1b����ôa:b=10:1��
��6��NH4Cl ��ҺΪ����Һ��pH=3����Һ�е�H+ȫ������ˮ���룬��c(H+)=10-3mol/L��
��7��Ksp��c(Fe3+)c3(OH-) ����c(Fe3��)=10��5 mol��L��1����c (OH-)=![]() =10-11mol/L��c (H+)=
=10-11mol/L��c (H+)=![]() =10-3mol/L��pH=3��������� c(Fe3��)<10��5 mol��L��1������pH����3��
=10-3mol/L��pH=3��������� c(Fe3��)<10��5 mol��L��1������pH����3��


 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��W��X��Y��Z��Q��ԭ���������������ǰ������Ԫ�ء�W����������ḻ��Ԫ�أ�X��Z Ԫ��ԭ�ӻ�̬ʱ��������Ӿ��Ų���3���ܼ��ϣ������ǵļ۵��Ӳ��Ͼ�������δ�ɶԵ��ӣ���Q2+����Һ�еμӰ�ˮ���γ���ɫ�������ٵμӰ�ˮ�������ܽ⣬�õ�����ɫ��Һ���ش��������⣺
��1����һ������ Y ________________ Z���縺�� Y ________________ Z����������������С������������������
��2��д����XZ���ӻ�Ϊ�ȵ������һ�����ӵĻ�ѧʽ________________��
��3��������Q2+ ����������Һ�еμӹ�����ˮ���õ�����ɫ��Һ���ټ��Ҵ�����_____________ɫ�����������þ����У��������ӵĵ����Ų�ʽΪ________________������Ϊ________________��
��4����֪ W �� Y �γɵ�һ���Ԫ�����ﻯѧʽ��![]() ��
��![]() ��
��![]() ��
��![]() ����
���У�![]() ������ Y ԭ�ӵ��ӻ�����Ϊ________________�����黯�����ͨʽΪ________________��W��Y �γɵĻ����������� W��X �γɵĻ����������________________ ������������������
������ Y ԭ�ӵ��ӻ�����Ϊ________________�����黯�����ͨʽΪ________________��W��Y �γɵĻ����������� W��X �γɵĻ����������________________ ������������������
��5��Q���ʵľ������������������ܶѻ�����֪ Q ���ʵ��ܶ��� ![]() ��NA��ʾ�����ӵ�������ֵ��Q �����ԭ������Ϊ M���� Q ���������ڽ���ԭ�Ӻ˼��Ϊ________________ cm �ú� M��d��NA �Ĵ���ʽ��ʾ ��Qԭ���ھ����еĿռ�������Ϊ________________���ú�N�Ĵ���ʽ��ʾ����
��NA��ʾ�����ӵ�������ֵ��Q �����ԭ������Ϊ M���� Q ���������ڽ���ԭ�Ӻ˼��Ϊ________________ cm �ú� M��d��NA �Ĵ���ʽ��ʾ ��Qԭ���ھ����еĿռ�������Ϊ________________���ú�N�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��£�ʯ����ﵽƽ�⣺Ca(OH)2 (s)![]() Ca2��(aq)��2OH��(aq)������������ȷ���ǣ� ��
Ca2��(aq)��2OH��(aq)������������ȷ���ǣ� ��
A.��ˮ��Ca(OH)2 �ܽ�ƽ�������ƶ���pH ����
B.�����������ᣬCa(OH)2 �ܽ�ƽ�������ƶ���ksp�zCa(OH)2�{����
C.���������������ƹ��壬Ca(OH)2 �ܽ�ƽ�������ƶ�����Һ�� c(H+)��С
D.�����¶ȣ�Ca(OH)2 �ܽ�ƽ�������ƶ���ksp�zCa(OH)2�{����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���Ԫ���� H2Y ��Һ�еμ� NaOH ��Һ�����û����Һ�� pH ������Ũ�ȱ仯�Ĺ�ϵ��ͼ��ʾ�������й�˵���� ����ǣ� ��
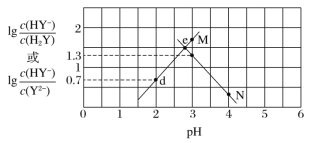
A.���� M ��ʾ pH �� lgc(HY-)/c(H2Y)�ı仯��ϵ
B.Ka2(H2Y)��10��4.3
C.d ����Һ�У�c(H��)��c(OH��)��2c(Y2��)��c(HY��)��c(Na��)
D.���� e ����Һ�У�c(H2Y)��c(Y2��)>c(HY��)>c(H��)>c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͼ1��ͭпԭ���ʾ��ͼ��ͼ2�У�x���ʾʵ��ʱ���������ĵ��ӵ����ʵ�����y���ʾ��������
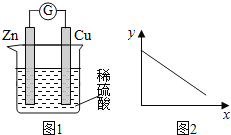
A. ͭ�������� B. c(Zn2��) C. c(H��) D. c(SO42-) -
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧѧϰ�г������Ʒ���������������ȷ����![]()
A.![]() Ϊֱ���η��ӣ�
Ϊֱ���η��ӣ�![]() ҲΪֱ���η���
ҲΪֱ���η���
B.![]() �ķе����
�ķе����![]() ��
��![]() �ķе�Ҳ����
�ķе�Ҳ����![]()
C.![]() ��Nԭ����
��Nԭ����![]() �ӻ���
�ӻ���![]() ��Bԭ��Ҳ��
��Bԭ��Ҳ��![]() �ӻ�
�ӻ�
D.![]() ������NaOH��Һ��
������NaOH��Һ��![]() Ҳ������NaOH��Һ
Ҳ������NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��µĶ����ܱ������У�������������������ʱ��仯ʱ��������A(s)+2B(g)![]() C(g)+D(g)һ���ﵽƽ��״̬����
C(g)+D(g)һ���ﵽƽ��״̬����
A.��������ѹǿB.���������ܶ�
C.�����������ʵ���D.���������C��D�����ʵ���֮��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȼ�ϵ����Ŀǰ����о����ȵ�֮һ������ij����С�����Ƶ�����ȼ�ϵ�أ���ͼ��ʾ��a��b��Ϊ���Ե缫��������������ȷ����(����)��
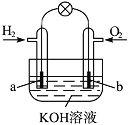
A.����ȼ�ϵ����һ�־���Ӧ��ǰ������ɫ��Դ
B.a���Ǹ������õ缫�Ϸ���������Ӧ
C.�ܷ�Ӧ����ʽΪ2H2��O2=2H2O
D.b����Ӧ��O2��4OH����4e��=2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����г����¼ס��ҡ���������Һ����Ϊ0.1 mol��L��1��NaOH��Һ����Ϊ0.1 mol��L��1��HCl��Һ����Ϊ0.1 mol��L��1��CH3COOH��Һ���Իش��������⣺
��1������Һ��pH��________��
��2������Һ�д��ڵĵ���ƽ��Ϊ______________(�õ���ƽ�ⷽ��ʽ��ʾ)��
��3�������£���ˮϡ��0.1 mol��L��1��CH3COOH��Һʱ�����и�����ˮ�������Ӷ��������________(�����)��
��n(H��)������������c(H��) �� c(CH3COOH)/c(CH3COO-) ��c(OH��)
��4���ס��ҡ���������Һ����ˮ�������c(OH��)�Ĵ�С��ϵΪ___________��
��5��ijͬѧ�ü���Һ�ֱ�ζ�20.00 mL����Һ��20.00 mL����Һ���õ���ͼ��ʾ�������ζ����ߣ���ش��й����⣺
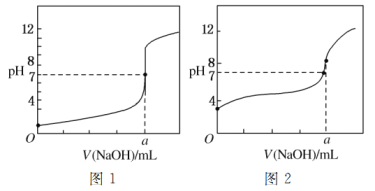
�ټ���Һ�ζ�����Һ��������________(����ͼ1������ͼ2��)���ߡ�
��a��________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com