
���� �ٵ�һ�ݼ���AgNO3��Һ�г���������˵�����ܺ���Cl-��CO32-��SO42-��
�ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�����ʹʪ���ɫʯ����ֽ���������壬֤��һ������NH4+���������ɣ�����Һ��һ������Mg2+��
�۵����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g��֤��ԭ��Һ��һ������CO32-��SO42-������CO32-��SO42-��һ��������Ba2+�����ɳ���BaSO4���ʵ���=$\frac{2.33g}{233g/mol}$=0.01mol�����ɵij���BaCO3���ʵ���=$\frac{6.27g-2.33g}{197g/mol}$=0.02mol����ϵ���غ������
��� �⣺�ٵ�һ�ݼ���AgNO3��Һ�г���������˵�����ܺ���Cl-��CO32-��SO42-��
�ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�����ʹʪ���ɫʯ����ֽ���������壬֤��һ������NH4+���������ɣ�����Һ��һ������Mg2+��
�۵����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g��֤��ԭ��Һ��һ������CO32-��SO42-������CO32-��SO42-��һ��������Ba2+�����ɳ���BaSO4���ʵ���=$\frac{2.33g}{233g/mol}$=0.01mol�����ɵij���BaCO3���ʵ���=$\frac{6.27g-2.33g}{197g/mol}$=0.02mol��
����������һ����NH4+��CO32-��SO42-��һ������Mg2+��Ba2+���ɵ���غ��֪����Һ�п��ܺ�K+��Cl-��
�ʴ�Ϊ��NH4+��CO32-��SO42-��Mg2+��Ba2+��K+��Cl-��
���� ���⿼�����ӵ��ƶϣ�Ϊ��Ƶ���㣬��������֮��ķ�Ӧ�����Ӽ��鷽��Ϊ���Ĺؼ������ط������ƶ������Ŀ��飬ע�����غ�Ӧ�ã���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
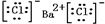 ���ݵĵ���ʽ��
���ݵĵ���ʽ��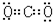 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | b+n | B�� | b+a+n | C�� | b+a-n | D�� | b-a+n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2O3$\frac{\underline{\;����\;}}{\;}$ 3O2 | |
| B�� | 2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$Al2O3+2Fe | |
| C�� | 3C12+2Fe $\frac{\underline{\;��ȼ\;}}{\;}$2FeCl3 | |
| D�� | 2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
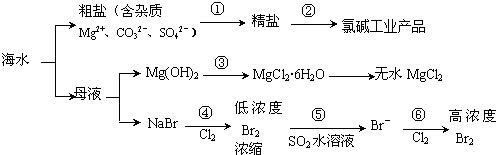
| A�� | ���̢���Ҫ���벻ֹ1���Լ�����ͨ�����ʵIJ��������ܰ����ʳ�ȥ | |
| B�� | ���̢ڵõ����ȼҵ��Ʒ��ֻ����1�ֵ��� | |
| C�� | ���̢۷������ֽⷴӦ | |
| D�� | ���̢ܡ��ݡ�������������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2O��g���TH2��g��+$\frac{1}{2}$O2��g����H=-242 kJ/mol | B�� | 2H2��g��+O2��g���T2H2O��l����H=-484 kJ/mol | ||
| C�� | H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H=-242 kJ/mol | D�� | 2H2��g��+O2��g���T2H2O��g����H=+484 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com