
����Ŀ�����з�Ӧ��mA(g)��nB(g) ![]() pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����
(1)�÷�Ӧ���淴ӦΪ______(��������������������)��Ӧ����m��n______(����>������������<��)p��
(2)��ѹʹ�����������ʱ��A����������________��(��������������С����������������ͬ)
(3)���ݻ��������B����A��ת����__________��B��ת����________��
(4)�������¶ȣ���ƽ��ʱB��C��Ũ��֮��![]() ��________��
��________��
(5)�����������ƽ��ʱ��������������ʵ���______________________��
���𰸡����� > ���� ���� ��С ��С ����
��������
��֪�ﵽƽ��������¶�ʱ��B��ת���ʱ���������¶ȣ�ƽ�������ƶ�������ӦΪ���ȣ�����Сѹǿ�������ʱ��ƽ�������������ķ����ƶ��������ϵ��C������������С��ƽ�������ƶ�����m+m>p��
��1�����ݷ�����֪������ӦΪ���ȣ����淴ӦΪ���ȷ�Ӧ��m��n>p��
��2����ѹ�ݻ�����ƽ����������ļ�����֮������ķ�Ӧ�����ƶ����������淴Ӧ�����ƶ�����A��������������
��3���ڷ�Ӧ�����м���һ������B����Ӧ��B��Ũ������ƽ��������Ӧ�����ƶ����ٽ�A��ת����A��ת��������B��ת���ʼ�С��
��4������Ӧ���ȣ��������¶�ƽ��������Ӧ�����ƶ���B�����ʵ���Ũ�ȼ�С��C�����ʵ���Ũ�����࣬����ߵ�Ũ�ȱ�ֵ����С��
��5�������Ի�ѧƽ���ƶ�û��Ӱ�죬�����������ƽ�ⲻ�ƶ�����ƽ��ʱ��������������ʵ������䡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ȷ���ǣ� ��
A.��0��Ԫ���⣬������Ԫ�ص���������ϼ�����ֵ�϶����ڸ�Ԫ���������������
B.���������⣬�������ھ�Ϊ18��Ԫ��
C.����Ԫ��û�зǽ���Ԫ��
D.�ڢ�B��������Ԫ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ������ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ���������·�ش�

��֪��CH3CHO + O2��CH3COOH
��1���������������������仯����__________������ţ���
�ٷ��� ���ѽ�
��2��A��������__________��
��3����ӦII�Ļ�ѧ����ʽ��________________��
��4��DΪ�߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ��________________��
��5��E������ζ�����ʣ���ӦIV�Ļ�ѧ����ʽ��______________________��
��6�����й���CH2��CH��COOH��˵����ȷ����__________��
����CH3CH��CHCOOH��Ϊͬϵ��
�ڿ�����NaHCO3��Һ��Ӧ�ų�CO2����
����һ�������¿��Է���ȡ�����ӳɡ�������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������I2O5������CO��Ⱦ����ӦΪI2O5(s)+5CO(g)![]() 5CO2(g)+I2(s)����ͬ�¶�������װ��������I2O5�����2L�����ܱ�������ͨ��2mol CO�����CO2 �����������(CO2)��ʱ��t�仯��������ͼ������˵����ȷ����
5CO2(g)+I2(s)����ͬ�¶�������װ��������I2O5�����2L�����ܱ�������ͨ��2mol CO�����CO2 �����������(CO2)��ʱ��t�仯��������ͼ������˵����ȷ����

A. b��ʱ��CO��ת����Ϊ20%
B. �����ڵ�ѹǿ���ֺ㶨��������Ӧ�ﵽƽ��״̬
C. b���d��Ļ�ѧƽ�ⳣ����Kb>Kd
D. 0��0.5min��Ӧ����v(CO)=0.3mol��L-1��min-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͨ��70�����������ˮ��Һ��,��ͬʱ������������������ԭ��Ӧ��δ��ƽ����NaOH+Cl2��NaCl+NaClO+H2O����Ӧ��ɺ�����Һ��NaClO��NaClO3����Ŀ֮��Ϊ5��2�������Һ��NaCl��NaClO����Ŀ֮��Ϊ( )
A.3��1B.2��1C.15��2D.1��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ܱ���ϵ�з������з�Ӧ��N2��g��+3H2��g��![]() 2NH3��g����H��0����ͼ��ʾ��ijһʱ����з�Ӧ�����뷴Ӧ���̵����߹�ϵͼ���ش��������⣺
2NH3��g����H��0����ͼ��ʾ��ijһʱ����з�Ӧ�����뷴Ӧ���̵����߹�ϵͼ���ش��������⣺

��1������ƽ��״̬��ʱ�����__��
��2��t1��t3��t4ʱ����ϵ�зֱ�ı����ʲô������
t1��__��t3��__��t4��__��
��3�����и�ʱ���ʱ���������������ߵ���______��
A��t2��t3 B��t3��t4 C��t4��t5 D��t5��t6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��GΪ��ѧ��ѧ�г��������ʣ�B��E��FΪ���ʣ��ڳ�����B��һ����ɫ���壬GΪ��ɫ���塣���ǵ��ת����ͼ��ʾ������������δ�г����Իش��������⣺
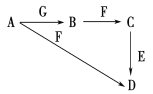
(1)F��һ�������ﳣ������ɫ�����Ϳ�ϣ�������������׳�Ϊ________��
(2)E�ڵ��������ӹ�ҵ��Ӧ����㣬Ҳ�����������Ľ���֮һ��д��E��C��Ӧ�����ӷ���ʽ________�����õ�ⷨ���ᴿE���ʣ��ڸõ�ⷴӦ������������________������������________���������Һ��__��
(3)д���������ʵĻ�ѧʽ��A________��G________��
(4)��B��SO2�������Ϻ�ͨ��Ʒ����Һ�У��۲쵽��������__________���漰�����ӷ���ʽΪ______����ҵ�ϻ��B����Ҫ;����________(�û�ѧ����ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С����0.50 mol��L��1 NaOH��Һ��0.50 mol��L��1 ������Һ�����к��ȵIJⶨ��

(1)�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ����������a������Ϊ_____________��
(2)д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ(�к���Ϊ57.3 kJ��mol��1)______________��
(3)ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
�� ����д�±��еĿհף�
�¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | __________ |
2 | 27.0 | 27.4 | 26.2 | 31.2 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�� ������Ϊ0.50 mol��L��1 NaOH��Һ��0.50 mol��L��1 ������Һ���ܶȶ���1 g��cm��3���кͺ�������Һ�ı�����c��4.18kJ��(kg����)��1�����к�����H��________________________��ȡС�����һλ����
�� ����ʵ�����ݽ����57.3 kJ��mol��1��ƫ� ����ƫ���ԭ�����ǣ�����ĸ��________��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��˫��ˮ��84����Һ���ճ������г��õ���������
��1��84����Һ����Ҫ�ɷ���NaClO)��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��_____________________��
��2��˫��ˮ��84����Һ���ʧȥ�������ã���������ɫ��ζ���壬�ڸ÷�Ӧ����������____________��
��3����ҵ�ϣ��Ʊ�84����Һ��ԭ���ǣ��Զ��Ե缫��ⱥ���Ȼ�����Һ�����������������ɵ��ռ���Һ���ա����������ĵ缫��ӦʽΪ_________________________��д���ܷ�Ӧ�Ļ�ѧ����ʽ______________��
��4��ʵ���ҷֱ���KMnO4��H2O2��KClO3�Ʊ�O2�����õ���������O2ʱ������Ӧ��ת�Ƶ��ӵ���Ŀ֮��Ϊ______________��
��5��˫��ˮ�Ƕ�Ԫ���ᣬ298 Kʱ��Ka1��1.6��1012��Ka2��1.0��1025����298 Kʱ��0.1 mol��L1˫��ˮ��Һ��pH��________________������֪��lg2��0.3��
��6����V2O5��������ϡ����õ�250mL(VO2)2SO4��Һ��ȡ25��00mL����Һ����ƿ�У���0��1000 mol��L-1H2C2O4����Һ���еζ����ﵽ�ζ��յ�ʱ���ı���Һ�����Ϊ20��00mL����֪�ζ�������H2C2O4������ΪCO2��VO2+(��ɫ)����ԭΪVO2+(��ɫ)��
�ٸõζ�ʵ�鲻��Ҫ�������ָʾ�����ﵽ�ζ��յ��������___________________��
��(VO2)2SO4��Һ�����ʵ����ʵ���Ũ��Ϊ___________________��
�۴ﵽ�ζ��յ�ʱ�����ӵζ��ܶ�����ʹ���_________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com