
����Ŀ��ʵ����Ҫ����100 mL 2 mol/L NaCl��Һ����ش��������⣺
(1)���ƹ�������Ҫʹ�õ���Ҫ�������������ձ�������������ͷ�ιܡ���Ͳ��___________��
(2)��������ƽ��ȡ�Ȼ��ƹ��壬������Ϊ__________ g��
(3)������Ҫ�����������ȷ˳����___________(�����)��
�ٳ�ȡһ���������Ȼ��ƣ������ձ��У�����������ˮ�ܽ⣻
�ڼ�ˮ��Һ��������ƿ���̶�����1~2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
�۽���Һת�Ƶ�����ƿ�У�
�ܸǺ�ƿ�����������µߵ���ҡ�ȣ�
������������ˮϴ���ձ��ڱںͲ�����2~3�Σ�ϴ��Һת�Ƶ�����ƿ�С�
(4)���ʵ�������ȱ�ٲ���ݣ������������Һ�����ʵ���Ũ��_______(����ƫ��������ƫ����������Ӱ������)
���𰸡�100mL����ƿ 11.7 �٢ۢݢڢ� ƫ��
��������
������Ҫ��������һ�����ʵ���Ũ�ȵ���Һʵ�顣
��1�������ù�������һ�����ʵ���Ũ����Һ��һ�㲽��ѡ����Ҫ��������
��2������m=cVM������Ҫ�Ȼ��Ƶ�������
��3�������ù�������һ�����ʵ���Ũ����Һ��һ�㲽������
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
��1���ù�������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ���������������ƽ��ҩ�ס��ձ���������������ƿ����ͷ�ιܣ�����100mL2mol/LNaCl��Һ��Ӧѡ��100mL����ƿ�����Ի�ȱ�ٵ�������100 mL����ƿ��
��2������100mL2mol/LNaCl��Һ����Ҫ���ʵ�����Ϊ��0.1L��2mol/L��58.5g/mol=11.7g��
��3���ù�������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ǩ��������ȷ�IJ�������Ϊ���٢ۢݢڢ���
��4�����ʵ�������ȱ�ٲ���ݣ�����ɲ���������ʧ�����ʵ����ʵ���ƫС��������Һ�����ʵ���Ũ��ƫ�͡�


 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������SO2��CO��NOx��Ⱦ�ǻ�ѧ�������о�����Ҫ���⡣
��.���᳧�����ŷź�SO2��β����Ի����������Σ����
��1����ҵ�Ͽ����÷ϼ�Һ����Ҫ�ɷ�ΪNa2CO3���������᳧β���е�SO2���õ�Na2SO3��Һ���÷�Ӧ�����ӷ���ʽΪ__________________________________��
��.�������������Ϊ��Ӧ��2CO(g)+O2(g)![]() 2CO2(g)�Ĵ�����ͼ�ױ�ʾ����ͬ�ĺ����ܱ���������ͬ��ʼŨ�ȡ���ͬ��Ӧʱ�����,ʹ��ͬ�����IJ�ͬ��������������͡����ͣ���ʱ��CO��ת�������¶ȵĹ�ϵ��
2CO2(g)�Ĵ�����ͼ�ױ�ʾ����ͬ�ĺ����ܱ���������ͬ��ʼŨ�ȡ���ͬ��Ӧʱ�����,ʹ��ͬ�����IJ�ͬ��������������͡����ͣ���ʱ��CO��ת�������¶ȵĹ�ϵ��

��2��a��b��c��d �ĵ��У��ﵽƽ��״̬����__________________________________��
��3����֪c��ʱ������O2Ũ��Ϊ0.02 mol/L����50��ʱ���ڦ��������������COת����Ӧ��ƽ�ⳣ��K=____________���ú�x�Ĵ���ʽ��ʾ����
��4�����й���ͼ��˵����ȷ����_____________��
A.COת����Ӧ��ƽ�ⳣ��K(a)
B.�ھ�δ�ﵽƽ��״̬ʱ��ͬ���¦��������������COת�����ʱȦ���Ҫ��
C.b��ʱCO��O2����֮�䷢����Ч��ײ�ļ���������ʵ����������
D.e��ת���ʳ���ͻ���ԭ��������¶����ߺ����ʧȥ����
��.ij���ܴ������Դ��������ͳ�β���е�̼��(C)��NOx����ͬ�¶��£���ģ��β�����ɷ����±���ʾ������ͬ������ͨ���ô�����������в��CO2��N2��N2O����NO��������ݽ����ͼ����ʾ��
ģ��β�� | ����(10mol) | ̼�� | ||
NO | O2 | He | ||
���ʵ���(mol) | 0.025 | 0.5 | 9.475 | n |
��5��375��ʱ������ų��������к�0.45 molO2��0.0525 mol CO2����Y�Ļ�ѧʽΪ______________��

��6��ʵ������в���NOģ��NOx����������NO2��ԭ����____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijϡ�����ϡ����Ļ����Һ200 mL��ƽ���ֳ����ݡ�������һ��������ͭ�ۣ��������19.2 g(��֪����ֻ����ԭΪNO����)������һ�����������ۣ�������������������������ӵı仯����ͼ��ʾ�����з�����������ȷ����

A. ͼ�У�AB�εķ�ӦΪFe��2Fe3��===3Fe2������Һ����������Ϊ��������
B. ԭ��Һ������Ũ��Ϊ2.5 mol��L��1
C. ԭ�����Һ����������ӵ����ʵ���Ϊ0.2 mol
D. ͼ�У�OA�β�����������һ��������BC�β���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ��ʵ���в�С�Ľ�����������ˮ����ʢ���廯������Һ���Լ�ƿ�У�������Һ����˻�ɫ���������л�ѧ֪ʶ������������������γ�ԭ��ķ�����̽����
��1�����������룺
����һ����Һ�ʻ�ɫ���������ӷ�Ӧ��_________________________________________(�����ӷ���ʽ)���¡�
���������Һ�ʻ�ɫ���������ӷ�Ӧ��______________________________________(�����ӷ���ʽ)���¡�
��2�����ʵ�鲢��֤
Ϊ��֤����������ĸ�ԭ��������Һ���ɫ����Ʋ�����������ʵ�顣��������������Լ������к���ѡ�ã����ʵ�鷽��1�ͷ���2��__________________��______________��__________________��______________��

��3��ʵ����ۣ�����ʵ�鲻����֤����Һ��Ƶ���ʵԭ��ͬʱ֤����Fe2���Ļ�ԭ�Ա�Br��______________(����ǿ����������)��
��4��ʵ�鷴˼
��.��������ʵ���Ʋ⣬�����廯������Һ�е���������ˮ���ټ���CCl4�������ֹ��������������______________________________________��
��.��100 mL FeBr2��Һ��ͨ��2.24 L Cl2(��״��)����Һ����1/2��Br���������ɵ���Br2����ԭFeBr2��Һ��FeBr2�����ʵ���Ũ��Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2017��5�º�����Ȼ��ˮ����(�׳ơ���ȼ����)�Բɳɹ��������ҹ���Դ������һ����ʷ��ͻ�ơ�һ�������£�CH4��CO2������H2O�γ�����ͼ��ʾ����״�ṹ(�����С����ˮ���ӣ��ڲ��Ĵ�����CH4���ӻ�CO2���ӣ�����ȼ������CH4��H2O�γɵ�ˮ����)������ز������±���
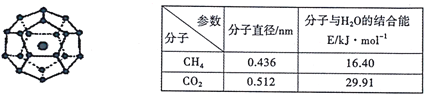
(1)CH4��CO2����������Ԫ�ص縺�ԴӴ�С��˳��Ϊ_______��̼ԭ�ӵ�����ܼ��ķ�����_______�����������״��_______��
(2)CO2������̼ԭ�ӵ��ӻ��������Ϊ_______�����ӵ����幹��Ϊ_____��CO2��SO2��ͬ��������ˮ�е��ܽ�Ƚϴ����SO2��������________��
(3)Ϊ���ɺ��ġ���ȼ�������п�ѧ�������CO2�û�CH4�����롣��֪��ͼ����״�ṹ�Ŀ�ǻֱ��Ϊ0.586nm����������ͼ���������ʽṹ�����ʵĽǶȷ������������������_______��
(4)����ȼ�����з��Ӽ���ڵ��������������_________����ͼ����С�Ļ������ӵ�ԭ��������_______��
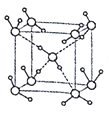
(5)ˮ�ڲ�ͬ���¶Ⱥ�ѹ�������¿��γ�11�ֲ�ͬ�ṹ�ľ��壬�ܶȴӱ�ˮ���0.92g/cm3��ԼΪˮ��1.5 ������������������֪����һ�ּ��Ӷѻ����ṹ�������Ļ�������б�- ���ľ���ṹΪһ����ͼ��ʾ������������ÿ��ˮ��������Χ4��ˮ�����������ϡ���O-H��O����Ϊapm�������ӵ�������ֵΪNA����ñ�- ��������ܶ�Ϊ____ g/cm3(�г�����ʽ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NAΪ�����ӵ�����������˵����ȷ����
A. ����Na2O2����ˮ��ַ�Ӧ������0.1mol O2�������ת����ĿΪ0.4NA
B. 1mol����(-NH2)�к��е��ӵ���ĿΪ9NA
C. 42g�л���C3H6�к���˫����ĿΪNA
D. 1mol/L��NaClO��Һ�к���ClO-����ĿС��NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��X��Y��Z��R��T��ԭ�Ӱ뾶��ԭ��������ϵ����ͼ��ʾ��Rԭ�������������ǵ��Ӳ�����2����Y��Z���γ�Z2Y��Z2Y2�����ӻ����Z��T�γɵĻ�����Z2T���ƻ�ˮ�ĵ���ƽ�⡣�����ƶ���ȷ����

A. ԭ�Ӱ뾶�����Ӱ뾶�����㣺Y<Z
B. �⻯��ķе㲻һ���ǣ�Y>R
C. ����������Ӧˮ��������ԣ�T<R
D. ��X��R��Y��Z����Ԫ����ɵĻ�����ˮ��Һһ���Լ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ca(OH)2�������һ����������ˮ�У�һ���¶��´ﵽƽ�⣺Ca(OH)2��s��![]() Ca2+��aq��+2OH����aq������������Һ�м�������ʯ�Һ����¶ȱ��ֲ��䣬�����ж���ȷ���� �� ��
Ca2+��aq��+2OH����aq������������Һ�м�������ʯ�Һ����¶ȱ��ֲ��䣬�����ж���ȷ���� �� ��
A. ��Һ��Ca2+��Ŀ���� B. ��Һ��c(Ca2+)����
C. ��ҺpHֵ���� D. ��ҺpHֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�ĸ������ʾ�йص�һ�ַ�Ӧ���������(ijЩ�����Ѿ���ȥ)�����г�����A��C��D��EΪ��ɫ���壬C��ʹʪ��ĺ�ɫʯ����ֽ������B������Ϊ��ɫҺ�壬Fe��Ũ��G��Һ�ۻ���

(1)д�����и����ʵĻ�ѧʽ��
B��____________��F��____________��G��___________��
(2)д�����б仯�ķ�Ӧ����ʽ��
A��D��________________________________________��
G��E��________________________________________��
(3)ʵ��������ü���_____________________�Ļ����ķ�����ȡ����C��������____________�����ռ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com