
ЃЈ1ЃЉбЧСђЫсФЦжагаЃЋ4МлЕФСђЃЌЫќМШгабѕЛЏадгжгаЛЙдадЃЌЯжгаЪдМСЃКфхЫЎЁЂNa2SШмвКЁЂ
Na2SO3ШмвКЁЂЯЁСђЫсЁЂNaOHШмвКЁЂАБЫЎЁЃ
ЃЈ1ЃЉвЊжЄУїNa2SO3ОпгаЛЙдадЃЌгІбЁгУЕФЪдМСга________ЃЌПДЕНЕФЯжЯѓЪЧ ________ЃЌЗДгІЕФЗНГЬЪНЮЊ___________________________
ЃЈ2ЃЉЪЕбщЪвжЦШЁТШЦјЕФЛЏбЇЗНГЬЪНЃК РЈКХжаЮЊдгжЪЃЌЬюЩЯГ§дгЪдМСЃКCl2жаЃЈHClЃЉ ЁЃ
ЃЈ3ЃЉгЩSiO2жЦШЁДжЙшЕФЛЏбЇЗНГЬЪН
ЃЈ4ЃЉНЋagгЩCOКЭH2зщГЩЕФЛьКЯЦјЬхдкзуСПЕФO2жаГфЗжШМЩеКѓЃЌНЋЩњГЩЕФЫљгаВњЮяЭЈЙ§зуСПЕФNa2O2ЙЬЬхЃЌNa2O2ЙЬЬхдіМгЕФжЪСПЮЊ ag ЃЈЃО ЃЛЃМ ЃЛЃНЃЉ
ЃЈ1ЃЉNa2SO3 фхЫЎ фхЫЎЭЪЩЋ Na2SO3+ Br2 + H2O = Na2SO4+2HBr
Na2SO3 + Br2 + H2O =H2SO4 + 2NaBr ЃЈ2ЃЉ MnO2 +4HCl(ХЈ)  MnCI2 + Cl2Ёќ+2H2O БЅКЭЪГбЮЫЎЃЈ3ЃЉSiO2 + 2C
MnCI2 + Cl2Ёќ+2H2O БЅКЭЪГбЮЫЎЃЈ3ЃЉSiO2 + 2C Si + 2CO ЃЈ4ЃЉ =
Si + 2CO ЃЈ4ЃЉ =
НтЮіЪдЬтЗжЮіЃКЃЈ1ЃЉвЊжЄУїNa2SO3ОпгаЛЙдадЃЌгІбЁгУЕФЪдМСЪЧNa2SO3КЭгаЧПЕФбѕЛЏадЕФЮяжЪЪЧфхЫЎЁЃЗЂЩњЕФЗДгІЪЧЃКNa2SO3+ Br2 + H2O = Na2SO4+2HBrЁЃЯжЯѓЮЊфхЫЎЭЪЩЋЁЃЃЈ2ЃЉ ЪЕбщЪвжЦШЁТШЦјЕФЛЏбЇЗНГЬЪНЃКMnO2 +4HCl(ХЈ)  MnCI2 + Cl2Ёќ+2H2OЁЃгЩгкЪЧгУЕФХЈбЮЫсЗДгІжЦШЁЕФЁЃХЈбЮЫсгаЛгЗЂадЃЌЫљвддкжЦШЁЕФCl2жаКЌгадгжЪHClЦјЬхЁЃГ§ШЅЕФЗНЗЈЪЧАбЛьКЯЦјЬхЭЈШыЕНБЅКЭNaClЫЎжаЃЌHClМЋШнвзШмНтдкЫЎжаЖјЕУЕНГ§ШЅЃЌЗЂЩњЗДгІCl2+H2O
MnCI2 + Cl2Ёќ+2H2OЁЃгЩгкЪЧгУЕФХЈбЮЫсЗДгІжЦШЁЕФЁЃХЈбЮЫсгаЛгЗЂадЃЌЫљвддкжЦШЁЕФCl2жаКЌгадгжЪHClЦјЬхЁЃГ§ШЅЕФЗНЗЈЪЧАбЛьКЯЦјЬхЭЈШыЕНБЅКЭNaClЫЎжаЃЌHClМЋШнвзШмНтдкЫЎжаЖјЕУЕНГ§ШЅЃЌЗЂЩњЗДгІCl2+H2O H++Cl-+HClO.вђЮЊЫЎжаКЌгаNaClЁЂМАHClЕчРыВњЩњЕФCl-ЃЌдіДѓСЫЩњГЩЮяЕФХЈЖШЃЌЪЙЛЏбЇЦНКтФцЯђвЦЖЏЃЌгжМѕЩйСЫCl2ЕФШмНтЁЂЗДгІЯћКФЁЃДяЕНСЫМШГ§ШЅдгжЪвВВЛМѕЩйБЛЬсДПЕФЮяжЪБОЩэЁЃЃЈ3ЃЉгЩSiO2жЦШЁДжЙшЕФЛЏбЇЗНГЬЪНЮЊSiO2 + 2C
H++Cl-+HClO.вђЮЊЫЎжаКЌгаNaClЁЂМАHClЕчРыВњЩњЕФCl-ЃЌдіДѓСЫЩњГЩЮяЕФХЈЖШЃЌЪЙЛЏбЇЦНКтФцЯђвЦЖЏЃЌгжМѕЩйСЫCl2ЕФШмНтЁЂЗДгІЯћКФЁЃДяЕНСЫМШГ§ШЅдгжЪвВВЛМѕЩйБЛЬсДПЕФЮяжЪБОЩэЁЃЃЈ3ЃЉгЩSiO2жЦШЁДжЙшЕФЛЏбЇЗНГЬЪНЮЊSiO2 + 2C Si + 2COЁЃЃЈ4ЃЉ2H2+O2
Si + 2COЁЃЃЈ4ЃЉ2H2+O2 2H2OЃЛ2Na2O2+2H2O=4NaOH+O2ЁќЃЛ2CO+O2
2H2OЃЛ2Na2O2+2H2O=4NaOH+O2ЁќЃЛ2CO+O2 2CO2ЁЃ2Na2O2+2CO2=2Na2CO3+O2ЁЃПЩМћЃКH2ЁЂCOШМЩеЯћКФЕФбѕЦјгыЗДгІВњЮягыNa2O2ЗДгІЗХГіЕФбѕЦјжЪСПЯрЕШЁЃМДЙЬЬхдіМгЕФжЪСПОЭЪЧЗДгІЮяH2ЁЂCOЕФжЪСПКЭЁЃШєЛьКЯЦјЬхЕФжЪСПЮЊagЃЌдђШМЩеВњЮягыЙ§бѕЛЏФЦЗДгІКѓЙЬЬхжЪСПдіМгЕФвВЪЧag.
2CO2ЁЃ2Na2O2+2CO2=2Na2CO3+O2ЁЃПЩМћЃКH2ЁЂCOШМЩеЯћКФЕФбѕЦјгыЗДгІВњЮягыNa2O2ЗДгІЗХГіЕФбѕЦјжЪСПЯрЕШЁЃМДЙЬЬхдіМгЕФжЪСПОЭЪЧЗДгІЮяH2ЁЂCOЕФжЪСПКЭЁЃШєЛьКЯЦјЬхЕФжЪСПЮЊagЃЌдђШМЩеВњЮягыЙ§бѕЛЏФЦЗДгІКѓЙЬЬхжЪСПдіМгЕФвВЪЧag.
ПМЕуЃКПМВщNa2SO3ЁЂNa2O2ЕФЛЏбЇаджЪМАSi ЁЂCl2ЕФжЦЗЈдгжЪЕФГ§ШЅЕШжЊЪЖЁЃ


 РјдХЪщвЕЪюМйЯЮНгФўВЈГіАцЩчЯЕСаД№АИ
РјдХЪщвЕЪюМйЯЮНгФўВЈГіАцЩчЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЕЅбЁЬт
ЯТСаЪЕбщЙ§ГЬжаЛсЯШВњЩњАзЩЋГСЕэКѓГСЕэгжШмНтЕФЪЧЃЈ ЃЉ
| AЃЎЯђAlCl3ШмвКжаЕЮМгАБЫЎжСЙ§СП |
| BЃЎЯђBa(NO3)2ШмвКЭЈШыSO2жСЙ§СП |
| CЃЎЯђBa(0H)2ШмвКЭЈШыCO2жСЙ§СП |
| DЃЎЯђаТжЦFe(OH)3НКЬхжаЕЮМгЯЁСђЫсжСЙ§СП |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЬюПеЬт
вбжЊXЁЂYЁЂZЁЂMЁЂQЁЂGЁЂRЁЂTЃЌЧАЫФжмЦкЕФ8жждЊЫиЃЈЮДАДеедзгађЪ§ХХСаЃЉЃЌZдЊЫиЕФФГжжЧтЛЏЮяПЩгУзїЛ№М§ЕФШМСЯЃЛQдЊЫиЕФЕЅжЪЮЊТЬЩЋжВЮяЙтКЯзїгУЕФвЛжжВњЮяЃЛИљОнЛЏбЇЖдНЧЯпЗЈдђЃЌGдЊЫиЖдНЧЯпЕФСэвЛжждЊЫиTЕФЕЅжЪПЩШмгкRдЊЫизюИпМлбѕЛЏЮяЫљЖдгІЫЎШмвКжаВЂЧвФПЧАвзРЙоАќКЌGдЊЫизюЖрЃЛYдЊЫиЕФМлЕчзгХХВМЪНЮЊЃЈn+1ЃЉSn ЃЈn+1ЃЉPЃЈn+3ЃЉЃЛ XдЊЫиФГжжЕФбЮШмвКПЩвдгыMдЊЫиЕФЕЅжЪЗЂЩњЗДгІЃЌВЂФмЩњГЩXдЊЫиЕФЕЅжЪЃЌЧвСНепКЫЕчКЩЪ§ЯрВю3ЃЌЧвMдЊЫиЕФЕЅжЪЪЧЕиПЧКЌСПЕкЖўИпЕФН№ЪєдЊЫиЃЛRдЊЫиЮЊЕкШ§жмЦкЕФФГжждЊЫиЁЃ
ЃЈ1ЃЉаДГіXдЊЫиЕФМлЕчзгХХВМЪН ЃЌZдЊЫиЖдгІЕФзюМђЕЅЧтЛЏЮяЕФПеМфЙЙаЭ ЁЃ
ЃЈ2ЃЉаДГідЊЫиTЕФЕЅжЪПЩШмгкRдЊЫизюИпМлбѕЛЏЮяЫљЖдгІЫЎШмвКЖдгІЕФРызгЗНГЬЪНЃК
ЁЃ
ЃЈ3ЃЉQдЊЫиЫљЖдгІЕФЧтЛЏЮяЕФЗаЕуБШЭЌжїзхдЊЫиЫљЖдгІЧтЛЏЮяЗаЕуИпЕФдвђЪЧЃК
ЁЃ
ЃЈ4ЃЉЩњЛюжаЃЌгІНЋКЌYЁЂQдЊЫиЕФФГжжЗлФЉзДЮяжЪУмЗтЁЂБмЙтЁЂИЩдяБЃДцЃЌЧвИУЗлФЉзДЮяжЪОГЃдкЯДвТЗўЪБгУЕНЃЌЧыгУЛЏбЇЗНГЬЪНЫЕУїЃЌИУЮяжЪГЈЗХдкПеЦјЪЇаЇЕФдвђЃК
ЁЃ
ЃЈ5ЃЉZдЊЫиЕФФГжжЧтЛЏЮяЃЌПЩгУзїЛ№М§ШМСЯЃЌЧвИУЛЏКЯЮяжаZдзггыЧтдзгЕФИіЪ§БШЮЊ1ЃК2ЃЌЙЄвЕЩЯгУФђЫиКЭЦЏАзвКЕФжївЊгааЇГЩЗжЃЌдкRдЊЫизюИпМлбѕЛЏЮяЫљЖдгІЫЎШмвКжаВЂгУИпУЬЫсМизїЮЊДпЛЏМСЗДгІЩњГЩетжжУћЮЊЫЎКЯАБЕФЛЏКЯЮяЃЌЩњГЩЕФЦфЫќВњЮяжаФГжжРызгЃЌПЩвдгыCaCl2ШмвКЗДгІЩњГЩФГжжАзЩЋГСЕэЃЌДѓРэЪЏжажївЊКЌетжжГЩЗжЁЃЭЈЙ§вдЩЯзЪСЯЃЌаДГіИУЛЏбЇЗНГЬЪНЃК
ЁЃ
ЃЈ6ЃЉШєШЁMдЊЫиЕФФГжжбѕЛЏЮягкЪдЙмжаЃЌМгШыЯЁбЮЫсЕїpHдМЮЊ7ЃЌМгШыЕэЗл-KIШмвККЭQдЊЫиЕФФГжжЛЏКЯЮяЃЌЧвИУЛЏКЯЮяЕФПеМфЙЙаЭЮЊвьУцелЯпаЮЃЌЗДгІЭъКѓШмвКГЪРЖЩЋВЂгаКьКжЩЋГСЕэЩњГЩЁЃЕБЯћКФ2mol I-ЪБЃЌЙВзЊвЦ3molЕчзгЃЌИУЗДгІЕФРызгЗНГЬЪНЃК
ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЬюПеЬт
АДвЊЧѓЬюПеЃК
ЃЈ1ЃЉГ§ШЅNaHCO3ШмвКжаЕФЩйСПNa2CO3ЃЌЗНЗЈЪЧЃК ЁЃ
ЃЈ2ЃЉКєЮќУцОпжаЫљгУЕФвЉЦЗЪЧ ЃЛЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃК ЁЃ
ЃЈ3ЃЉгЩТСбЮжЦШЁЧтбѕЛЏТСЃЌЫљгУЕФЪдМСЮЊ ЃЛРызгЗНГЬЪНЮЊЃК ЁЃ
ЃЈ4ЃЉаДГіЙшЫсФЦШмвКдкПеЦјжаБфжЪЕФЛЏбЇЗНГЬЪНЃК ЁЃ
ЃЈ5ЃЉаДГігУЪьЪЏЛвЮќЪеТШЦјжЦЦЏАзЗлЕФЛЏбЇЗНГЬЪНЃК ЁЃ
ЃЈ6ЃЉНЋCaMg3Si4O12ИФаДЮЊбѕЛЏЮяЕФаЮЪНЃК______________________________________________ЁЃ
ЃЈ7ЃЉаДГіЪЕбщЪвжЦШЁТШЦјЕФЛЏбЇЗНГЬЪН_____________________________ЁЃЗДгІзЊвЦЕчзгзмЪ§ЮЊ____ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЬюПеЬт
аДГіЯТСаГ§ШЅдгжЪЫљгУЕФЪдМСКЭЖдгІЕФРызгЗНГЬЪНЃК
ЃЈ1ЃЉFeCl2ШмвКЛьгаFeCl3дгжЪЃЌГ§дгЪдМС___________ЃЌРызгЗНГЬЪН_________________________ ЁЃ
ЃЈ2ЃЉCO2ЦјЬхЛьгаHClдгжЪЃЌГ§дгЪдМС_____________ЃЌРызгЗНГЬЪН____________________________ЁЃ
ЃЈ3ЃЉNaHCO3ШмвКЛьгаNa2CO3дгжЪЃЌГ§дгЪдМС________ЃЌРызгЗНГЬЪН___________________________ЁЃ
ЃЈ4ЃЉNOжаЛьгаNO2дгжЪЃЌГ§дгЪдМС_____________ЃЌРызгЗНГЬЪН_______________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЬюПеЬт
XЁЂYЁЂZЁЂQЁЂRЪЧЮхжжЖЬжмЦкдЊЫиЃЌдзгађЪ§вРДЮдіДѓЁЃXЁЂYСНдЊЫизюИпе§МлгызюЕЭИКМлжЎКЭОљЮЊ0ЃЛQгыXЭЌжїзхЃЛZЁЂRЗжБ№ЪЧЕиПЧжаКЌСПзюИпЕФЗЧН№ЪєдЊЫиКЭН№ЪєдЊЫиЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЮхжждЊЫидзгАыОЖгЩДѓЕНаЁЕФЫГађЪЧЃЈаДдЊЫиЗћКХЃЉ ЁЃ
ЃЈ2ЃЉXгыYФмаЮГЩЖржжЛЏКЯЮяЃЌЦфжаМШКЌМЋадМќгжКЌЗЧМЋадМќЃЌЧвЯрЖдЗжзгжЪСПзюаЁЕФЮяжЪЃЈаДЗжзгЪНЃЉ ЁЃ
ЃЈ3ЃЉгЩвдЩЯФГаЉдЊЫизщГЩЕФЛЏКЯЮяAЁЂBЁЂCЁЂDгаШчЯТзЊЛЏЙиЯЕ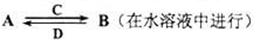
ЦфжаCЪЧШмгкЫЎЯдЫсадЕФЦјЬхЃЛDЪЧЕЛЦЩЋЙЬЬхЁЃаДГіCЕФНсЙЙЪН ЃЛDЕФЕчзгЪН ЁЃ
ЂйШчЙћAЁЂBОљгЩШ§жждЊЫизщГЩЃЌBЮЊСНадВЛШмЮяЃЌдђAЕФЛЏбЇЪНЮЊ ЃЛ
гЩAгыЙ§СПЕФCЗДгІзЊЛЏЮЊBЕФРызгЗНГЬЪН ЁЃ
ЂкШчЙћAгЩШ§жждЊЫизщГЩЃЌBгЩЫФжждЊЫизщГЩЃЌAЁЂBШмвКОљЯдМюадЁЃгУРызгЗНГЬЪНБэЪОAШмвКЯдМюадЕФдвђ ЁЃAЁЂBХЈЖШОљЮЊ0ЃЎ1mol/LЕФЛьКЯШмвКжаЃЌРызгХЈЖШгЩДѓЕНаЁЕФЫГађЪЧ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЬюПеЬт
ФГЭЌбЇЩшМЦШчЭМзАжУЃЌбаОПЗЧН№ЪєдЊЫиаджЪБфЛЏЙцТЩ.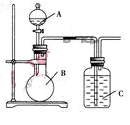
ЃЈ1ЃЉвбжЊЙшЫс(H2SiO3)ЪЧвЛжжФбШмгкЫЎЕФШѕЫсЃЌГЪАзЩЋЁЃдкЛЏбЇЗДгІжаЃЌвЛАуЕиЃЌЧПЫсФмжЦШѕЫсЃЌШчNaHCO3ЃЋHCl=NaClЃЋCO2ЁќЃЋH2OЃЌЕУГіЃКHClЫсадЧПгкH2CO3ЕФЫсадЁЃЯжгаЯѕЫсШмвКЁЂЬМЫсИЦЁЂГЮЧхЪЏЛвЫЎЁЂЙшЫсФЦШмвКЃЌбЁдёЪдМСгУШчЭМзАжУжЄУїЃКЫсадЃКHNO3>H2CO3>H2SiO3ЁЃ
AжазАЪдМС________ЃЌBжазАЪдМС____________ЃЌCжазАЪдМС____________ЁЃCжаЪЕбщЯжЯѓЮЊ____________ЃЛаДГіCжаЗЂЩњЗДгІЕФРызгЗНГЬЪН_____________________________ЁЃ
ЃЈ2ЃЉвбжЊИпУЬЫсМидкГЃЮТЯТгыХЈбЮЫсЗДгІВњЩњТШЦјЃЌРћгУШчЭМзАжУжЄУїТШЦјбѕЛЏадЧПгкЕтЕЅжЪЕФбѕЛЏадЁЃдђAжазАХЈбЮЫсЃЌBжазАШыИпУЬЫсМиЗлФЉЃЌCжазАЪдМС________ЃЌCжаЯжЯѓ________ЃЌаДГіРызгЗНГЬЪН__________________ЁЃИУЪЕбщзАжУгаУїЯдВЛзуЃЌЧыжИГіИФНјЗНЗЈЃК_______________________________________ЁЃ
ЃЈ3ЃЉШчЙћCжазАБЅКЭЕФЧтСђЫсШмвКЃЌAжазАХЈбЮЫсЃЌBжазАИпУЬЫсМиШмвКЃЌЗДгІПЊЪМКѓЙлВьЕНЯжЯѓЪЧCжаВњЩњЕЛЦЩЋГСЕэЃЌаДГіЛЏбЇЗНГЬЪН______________________________ЃЛжЄУїТШЕФЗЧН№ЪєадБШСђЕФЗЧН№Ъєад________(ЬюЁАЧПЁБЛђЁАШѕЁБЛђЁАЮоЗЈХаЖЯЁБ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЬюПеЬт
NaClOКЭKAl(SO4)2ЖМЪЧживЊЕФЛЏЙЄВњЦЗЃЌОљПЩгІгУгкдьжНвЕЁЃ
ЃЈ1ЃЉЙЄвЕЩЯПЩгУТШЛЏФЦЮЊдСЯЃЌЭЈЙ§ЕчНтЕФЗНЗЈжЦЕУNaClOЃЌЙЄвЕЩЯжЦШЁNaClOЕФРызгЗДгІЗНГЬЪНЮЊ
ЕчНтКѓЕФШмвКPH 7ЃЈЬюДѓгкЁЂаЁгкЁЂЕШгкЃЉЃЛЦфдвђЪЧ ЃЈгУРызгЗНГЬЪНБэЪОЃЉ
ЃЈ2ЃЉKAl(SO4)2ШмвКжажЪзгЪиКуЕШЪНЮЊ
ЃЈ3ЃЉФГаЁзщЭЌбЇгУЯТЭМЫљЪОзАжУЬНОПБЅКЭNaClOКЭKAl(SO4)2ШмвКЛьКЯЗДгІЕФЪЕбщЁЃ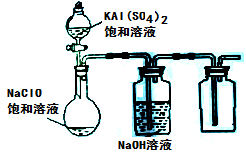
ЂйДђПЊЛюШћЯђЩеЦПжаМгШыБЅКЭKAl(SO4)2ШмвКЃЌВњЩњДѓСПЕФАзЩЋНКзДГСЕэЁЃДЫЪБЗДгІЕФРызгЗНГЬЪНЮЊ
ЁЃ
ЂкНЋЩеЦПжаЕФЛьКЯвКдкбєЙтееЩфЯТЃЌВЛОУЩеЦПжагаЛЦТЬЩЋЦјЬхВњЩњЃЌГфЗжЗДгІКѓМЏЦјЦПжаЪеМЏЕНвЛжжЮоЩЋЮоЮЖЕФЦјЬхЁЃаДГідкЙтееЩфЯТЛьКЯвКжаЗДгІЕФЛЏбЇЗНГЬЪНЪЧ ЁЃ
ЃЈ4ЃЉШєНЋЗжвКТЉЖЗжаЕФKAl(SO4)2ШмвКЛЛГЩСђЫсбЧЬњяЇЃЈвЛжжИДбЮ:(NH4)2SO4ЁЄFeSO4ЃЉШмвК,ЦфЫћВЛБфЁЃДђПЊЗжвКТЉЖЗЛюШћЯђЩеЦПжаЕЮШызуСПЕФСђЫсбЧЬњяЇШмвКЁЃЙлВьЕНЩеЦПжагаКьКжЩЋГСЕэВњЩњЃЌЕЋЪЧУЛгаЙлВьЕНЛЦТЬЩЋЦјЬхВњЩњЁЃДЫЪБЩеЦПжаЗЂЩњЕФбѕЛЏЛЙдЗДгІЕФРызгЗНГЬЪНЮЊ
ЁЃ
ЃЈ5ЃЉШЁ100mL 0.1mol/L Ba(OH)2ШмвКЃЌЯђЦфжаж№ЕЮМгШыЭЌХЈЖШЕФKHSO4ШмвКжСBa2+ЧЁКУЭъШЋГСЕэЃЌДЫЪБШмвКЕФPHжЕЮЊ ЃЈКіТдСНШмвКЛьКЯЪБЕФЬхЛ§БфЛЏЃЌЛьКЯКѓШмвКЕФЮТЖШЮЊ100ЁцЃЌ100ЁцЪБKw=1x10-12)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЕЅбЁЬт
ШЫУЧГЃНЋдкЭЌвЛдзгЙьЕРЩЯдЫЖЏЕФЃЌзда§ЗНЯђЯрЗДЕФ2ИіЕчзгЃЌГЦЮЊЁАЕчзгЖдЁБЃЛНЋдкЭЌвЛдзгЙьЕРЩЯдЫЖЏЕФЕЅИіЕчзгЃЌГЦЮЊЁАЮДГЩЖдЕчзгЁБЁЃвдЯТгаЙижїзхдЊЫидзгЕФЁАЮДГЩЖдЕчзгЁБЕФЫЕЗЈЃЌДэЮѓЕФЪЧ( )
ЂйЦцЪ§зхдЊЫиЕФЛљЬЌдзгЃЌЦфдзгЙьЕРжавЛЖЈКЌгаЁАЮДГЩЖдЕчзгЁБ
ЂкХМЪ§зхдЊЫиЕФЛљЬЌдзгЃЌЦфдзгЙьЕРжавЛЖЈВЛКЌЁАЮДГЩЖдЕчзгЁБ
ЂлХМЪ§зхдЊЫиЕФЛљЬЌдзгЃЌЦфдзгЙьЕРжаПЩФмКЌгаЁАЮДГЩЖдЕчзгЁБ
ЂмЦцЪ§зхдЊЫиЕФЛљЬЌдзгЃЌЦфдзгЙьЕРжаПЩФмВЛКЌЁАЮДГЩЖдЕчзгЁБ
| AЃЎЂйЂл | BЃЎЂкЂм | CЃЎЂк | DЃЎЂм |
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com