
����Ŀ��Al��Mg���仯���������������������Ź㷺��Ӧ�ã��밴Ҫ��ش��������⡣
I. �÷������Ʊ�Al(OH)3����������ͼ��ʾ�����跴Ӧ�����ʲ���Ӧ��

��1��Al��Ԫ�����ڱ��е�λ��Ϊ______���Լ�X������______ ��
��2����ҺA�����Ե�ԭ�������(�������ӷ���ʽ��ʾ) a.���������b. ____________��
II.��þ�����ο�ʯ�Ʊ��ߴ���Mg(OH)2����������ͼ��ʾ:
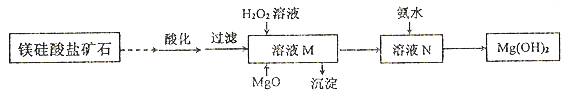
��֪������ҺM�г���Mg2+��SO42-�⣬��������Fe3+�� Al3+��Fe2+��������
��Mg2+�백ˮ�ķ�ӦΪ���ȷ�Ӧ��
��1������ҺM���ȼ�����Լ���_______�������ij�����_____��H2O2���뷴Ӧ�����ӷ���ʽ��____________________��
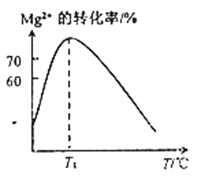
��2����ҺN��Mg2+��װ�������¶�T�ı仯�����ͼ��ʾ��T1֮����Mg,2+��ת���ʼ�С��ԭ����__________________��
���𰸡� �������ڵ�IIIA�� ��ˮ Al3++3H2O![]() Al(OH)3+3H+ H2O2��Һ Al(OH)3��Fe(OH)3 2Fe2++2H++H2O2=2Fe3++2H2O ��ϵ�¶����ߣ�NH3��H2O�ֽ����Ũ�Ƚ��ͣ���Mg2+ת���ʽ���
Al(OH)3+3H+ H2O2��Һ Al(OH)3��Fe(OH)3 2Fe2++2H++H2O2=2Fe3++2H2O ��ϵ�¶����ߣ�NH3��H2O�ֽ����Ũ�Ƚ��ͣ���Mg2+ת���ʽ���
��������I.��1��AlΪ13��Ԫ�أ���������Ų�Ϊ1S22S22P63S23P1,�����ڱ��е�λ��Ϊ�������ڵ�IIIA�塣��ΪAl(OH)3�����ڹ�����ǿ�������������Ը���ͼ�����Լ�XӦΪ��ˮ��
��2����Ϊ��ҺA�к��е�����ΪAl3+��Mg2+�������������������������Һ�����Ե�ԭ�������a.���������Ҳ������Al3+����ˮ�ⷴӦ�ˣ��䷽��ʽΪAl3++3H2O![]() Al(OH)3+3H+��
Al(OH)3+3H+��
II.��1����Ϊ����ҺM�г���Mg2+��SO42-�⣬��������Fe3+��Al3+��Fe2+�����ӣ�Ϊ�˳�ȥFe3+��Al3+��Fe2+Ӧ�Ȱ�Fe2+������Fe3+���ֲ������������ʣ���������ҺM���ȼ���H2O2��Һ����Fe2+���䷴Ӧ����ʽΪ��2Fe2++2H++H2O2=2Fe3++2H2O����MgO������Һ������ԣ�ʹFe3+��Al3+����Al(OH)3��Fe(OH)3�ij������𰸣�H2O2��Һ Al(OH)3��Fe(OH)3 2Fe2++2H++H2O2=2Fe3++2H2O��
��2����Mg2+ת�������¶�t�ı仯ʾ��ͼ����֪������Ϊ��ˮ��þ���ӷ�Ӧ����������þ�ķ�Ӧ�����ȷ�Ӧ�����������¶ȣ�þ���ӵ�ת�������������¶ȵ�����NH3��H2O�ֽ����Ũ�Ƚ�����������ˮ�е��ܽ�ȼ�С����ˮ��Ũ�ȼ�С������T1֮��ᵼ��Mg2+ת���ʼ�С���𰸣���ϵ�¶����ߣ�NH3��H2O�ֽ����Ũ�Ƚ��ͣ���Mg2+ת���ʽ�����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.(��ѡ)�����Ƶ�±����(NaX)���±����(SiX4)������������ȷ������____��
A��SiX4��ˮ�� B��SiX4�ǹ��ۻ�����
C��NaX��ˮ�� D��NaX���۵�һ�����SiX4
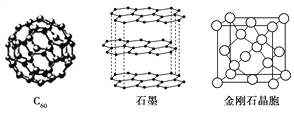
��.̼Ԫ�صĵ����ж�����ʽ����ͼ������C60��ʯī�ͽ��ʯ�Ľṹͼ��
�ش��������⣺
(1)���ʯ��ʯī��C60��̼���ܵȶ���̼Ԫ�صĵ�����ʽ�����ǻ�Ϊ________��
(2)���ʯ��ʯīϩ(ָ����ʯī)��̼ԭ�ӵ��ӻ���ʽ�ֱ�Ϊ________��________��
(3)C60����________���壬ʯī����________���塣
(4)ʯī�����У�����C��C���ļ���Ϊ142 pm�������ʯ��C��C���ļ���Ϊ154 pm����ԭ���ǽ��ʯ��ֻ����C��C���________���ۼ�����ʯī���ڵ�C��C�䲻������________���ۼ�������________����
(5)���ʯ��������________��̼ԭ�ӡ���̼ԭ�Ӱ뾶Ϊr�����ʯ�����ı߳�Ϊa������Ӳ��Ӵ�ģ�ͣ���r��________a����ʽ��ʾ̼ԭ���ھ����еĿռ�ռ����________(��Ҫ�������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ�������������������Ԫ��X��Y��Z��W�ֱ��ڵ�һ���������ڣ���Ȼ���д��ڶ���X�Ļ����Yԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��Y��Z���γ����������η��ӣ�W�Ļ�̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӡ���ش��������⣺
��1��������Ԫ���е縺������Ԫ�أ����̬ԭ�ӵļ۵����Ų�ͼΪ_________��
��2��Z�������������Ԫ�طֱ���X�γɵĻ�����е��ɸߵ��͵�˳����______���ѧʽ����������˵ݱ���ɵ�ԭ����________________________________��
��3��YԪ�ؿ��γɶ��ֵ��ʣ�һ�־���ṹ��ͼһ��ʾ����ԭ�ӵ��ӻ�����Ϊ___����һ�ֵľ�����ͼ����ʾ���þ����Ŀռ�������Ϊ________________��������λ��Ч���֣�����![]() ��
��
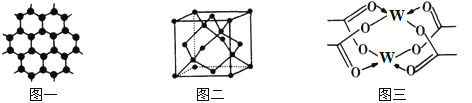
��4��WԪ���γɵĵ��ʣ��侧��Ķѻ�ģ��Ϊ___________��W�Ĵ����ξ���ֲ��ṹ��ͼ�����þ����к��еĻ�ѧ����____________(��ѡ�����)��
�ټ��Լ� �ڷǼ��Լ� ����λ�� �ܽ�����
��5����W����������Һ�еμӹ�����ˮ���۲쵽��������____________����д���������̵����ӷ���ʽ___________________________ ��___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£�CH4��H2O(g)������Ӧ��CH4(g)+H2O(g)![]() CO(g)+3H2(g)������ʼ
CO(g)+3H2(g)������ʼ![]() =Z���ں�ѹ�£�ƽ��ʱ
=Z���ں�ѹ�£�ƽ��ʱ![]() (CH4)�����������Z��T(�¶�)�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
(CH4)�����������Z��T(�¶�)�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
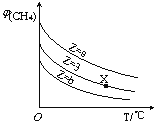
A���÷�Ӧ���ʱ��H>0
B��ͼ��Z�Ĵ�СΪa>3>b
C��ͼ��X���Ӧ��ƽ��������![]() =3
=3
D���¶Ȳ���ʱ��ͼ��X���Ӧ��ƽ���ڼ�ѹ��![]() (CH4)��С
(CH4)��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ����
A. ��������������������Һ��Al+2OH��=AlO2��+H2��
B. ����ˮ�ķ�Ӧ��Na+2H2O=Na++2OH��+H2��
C. ͭƬ��ϡ����ķ�Ӧ��Cu+NO3��+4H+=Cu2++NO��+2H2O
D. ������ͨ���Ȼ�������Һ��2Fe2++Cl2=2Fe3++2Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ư����Һ���ݹ�����ɫ����������ɹ�ڿ����У���һ��ʱ�䣬��Ư��Ч�����õ�ԭ���ǣ�������
A.Ư�۱�������
B.Ư�ۺͿ����еĶ�����̼��ַ�Ӧ�����˴�����
C.��ɫ�����������е���������
D.Ư����Һʧȥ�˲���ˮ�֣�Ũ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ƣ�Na2S2O4���׳Ʊ��շۣ��ױ�����������������ͼװ�ã�����ƿ�м���HCOONa��NaOH��CH3OH��ˮ�γɵĻ��Һ��ͨ��SO2ʱ������Ӧ���ɱ��շۺ�һ�ֳ������壬����˵���������
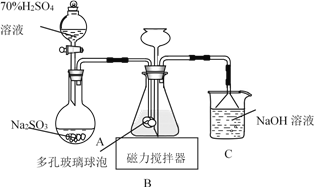
A. �Ʊ����շ۵����ӷ���ʽΪHCOO����OH����2SO2===S2O42����CO2����H2O
B. NaOH��Һ����Ҫ�����������ݳ���CO2
C. ��ײ������ݵ�������������������Һ�ĽӴ������ʹSO2�ܱ��������
D. Ϊ���������Na2S2O4��O2������ʹ���������������ȷ�Ӧ��������SO2�ų�װ���в�����O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��д�������������ӷ���ʽ�Ļ�ѧ����ʽ��Ba2++SO42-=BaSO4��________________��
��2�������ֽ���������ˮ��ԭ��______________________________________________��
��3�����ݷ�Ӧ8NH3+3Cl2=6NH4Cl+N2���ش��������⣺
���õ����ű�ʾ���÷�Ӧ����ת�Ƶ���Ŀ�ͷ���_______________________��
����������_____________��
�۵���34gNH3�μӷ�Ӧʱ�������������ʵ�����Ϊ_____g�����ɵĻ�ԭ���������Ϊ____g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ��̽�������ڸ�������ˮ������Ӧ��ʵ�顣
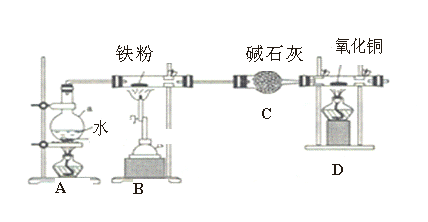
(1)Aװ�ü���������___________________________��
(2)B�з�Ӧ�Ļ�ѧ����ʽ��_________________________________________����״���£����÷�Ӧ����22.4L����,��Ӧת�Ƶĵ�����Ϊ______________��
(3)װ��D�е�������___________________,д��D�з�Ӧ����ʽ��_________________��
(4)A ��B�����ƾ��Ƶ�ȼ˳��____________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com