
ij��ѧС���������������������װ�ã���ͼ�����Ի�����Ϊ��Ҫԭ���Ʊ�����ϩ��
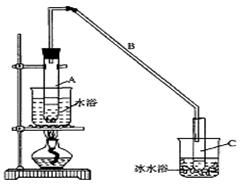
(1)�Ʊ���Ʒ
��12.5 mL�����������Թ�A�У��ټ���l mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������____________������B���˵�������е�������____________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����______________________________��
(2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_________��(���ϻ���)����Һ����_________ (������)ϴ�ӡ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��_________�ڽ���(�g����f��)���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����________________________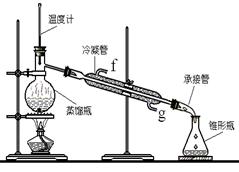
��1���ٷ�ֹ���ҷ��� ���� ��ʹ����ϩҺ�������ٻӷ�
��2�����ϲ� C ��g ��83 ��
�Ʊ���Ʒʱ���������Ʒһ������
���������������1����A�����Ƭ�������Ƿ�ֹ���У�����B���˵�������е�������������ʹ����ϩҺ����
�ڻ���ϩ���۷е�ͣ������Թ�C���ڱ�ˮԡ�е�Ŀ����ʹ����ϩҺ�������ٻӷ���
��2���ٻ���ϩ���ܶȱ�ˮС�����Լ��뱥��ʳ��ˮ�������á��ֲ㣬����ϩ���ϲ㣻��������д�������ϴ�ӣ��ױ����������������������ʣ�������ϡH2SO4������ѡ��̼������Һ���������е��������ʷ�Ӧ����ѡC��
������ʱ��ȴˮ���¶˽����϶˳���������ȴˮ��g�ڽ�������ϩ�ķе���83�棬���Կ��Ƶ��¶�Ӧ��83�����ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ�����Ʊ���Ʒʱ���������Ʒһ��������ʹ���ʽ��͡�
���㣺�����ʵ��װ�����õ��жϣ���ʵ�����ķ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ȥ���������������������ʣ���д��ѡ�õ��Լ��ͷ��뷽��
| | ����� (������Ϊ��������) | �Լ� �������� | ���뷽�� |
| A | �������ӣ� | | |
| B | ��ϩ��SO2�� | | |
| C | �������������ᣩ | | |
| D | �Ҵ���ˮ�� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������Ӱ�����ǵ������뽡����ij�����������п��ܺ������¿����������ӣ�Na+��NH4+��Mg2+��Al3+��SO42����NO3����Cl�� ��ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ������������Һ����Ʋ���������µ�ʵ�飺
��֪��3NO3��+ 8Al + 5OH�� + 2H2O 3NH3 + 8AlO2��
3NH3 + 8AlO2��
�������ϵ�ʵ�����������ͬѧ�ó��Ľ��۲���ȷ����
�����п϶�����NH4+��Mg2+��SO42����NO3��
������һ������Al3+
�����п��ܴ���Na+��Cl��
�������п��ܴ���NaNO3��NH4Cl��MgSO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣�CoCl2��6H2O��һ������Ӫ��ǿ������һ������ˮ�����Ҫ�ɷ�ΪCO2O3��Co
��OH��3����������Fe2O3��Al2O3��MnO�ȣ���ȡCoCl2��6H2O�Ĺ����������£�
��֪���ٽ���Һ���е���������Ҫ��H+��CO2+��Fe2+��Mn2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±�������������Ũ��Ϊ��0��01 mo1��L-l��
��CoCl2��6H2O�۵�Ϊ86�棬������110��120��ʱ��ʧȥ�ᾧˮ������ˮ�Ȼ��ܡ�
��1��д������������Co2O3������Ӧ�����ӷ���ʽ ��
��2��д��NaC1O3������Ӧ����Ҫ���ӷ���ʽ ������������Һ���мӹ���NaClO3ʱ�����ܻ������ж����壬д�����ɸ��ж���������ӷ���ʽ ��
��3������Na2CO3��pH��a'�����������õ��ij����ɷ�Ϊ____ ��
��4����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ������Һ���м�����ȡ����Ŀ���� ����ʹ�õ����pH��Χ��____ ��
| A��2��0��2��5 | B��3��0��3��5 | C��4��0��4��5 | D��5��0��5��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ��������������ˮ���������Ҵ��Ʊ�1��2�����������װ������ͼ��ʾ��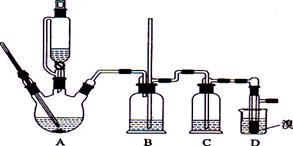
�й������б����£�
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g �� cm-3 | 0.79 | 2.2 | 0.71 |
| �е㣯�� | 78.5 | 132 | 34.6 |
| �۵㣯�� | -l30 | 9 | -1l6 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
1,2����������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ���2��18g/cm3���е�131��4�棬�۵�9��79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1,2-�������顣���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Һ��(���渲������ˮ)��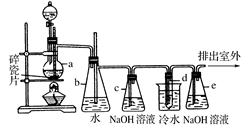
(1)д���������Ʊ�1,2-���������������ѧ��Ӧ����ʽ_____________________________��
(2)��ȫƿb���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е�����________________________________________��
(3)����c��NaOH��Һ�������ǣ� ��
(4)ijѧ������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ��������������³������࣬���װ�õ�������û�����⣬�Է�������ܵ�ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����״���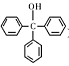 ����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���������ͼ1��ʾ��װ����ͼ��ʾ��
����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���������ͼ1��ʾ��װ����ͼ��ʾ��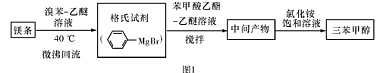
��֪����I�������Լ�����ˮ�⣺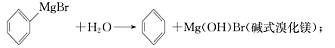
����������ʵ������������£�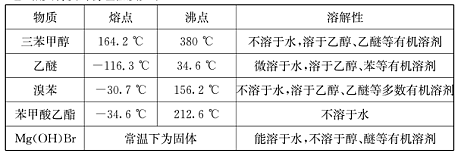
���������״�����Է���������260�����������л���һ�㶼�й̶��۵㡣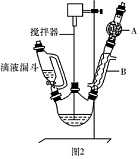
��ش��������⣺
��1��ͼ2�в�������B�����ƣ� ��װ����ˮCaCl2������A�������� ��
��2��ͼ2�еμ�Һ��δ����ͨ��Һ©�����õ�Һ©���������� ����ȡ�����Լ�ʱҪ�����У����Բ��� ���ȷ�ʽ��
��3���Ƶõ������״��ֲ�Ʒ�У��������ѡ��屽���������������л���ͼ�ʽ�廯þ�����ʣ�������������ᴿ����������д���¿հף�
���У��ٲ���Ϊ�� ��ϴ��Һ���ѡ�� ��������ѡ����ѡ��
| A��ˮ | B������ | C���Ҵ� | D���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������泥�NH2COONH4����һ�ְ�ɫ���壬�ֽ⡢��ˮ�⣬���������ϡ�������ϴ�Ӽ��ȡ�ij��ѧ��ȤС��ģ�ҵԭ���Ʊ���������泥���Ӧ�Ļ�ѧ����ʽ���£�
2NH3��g����CO2��g��??NH2COONH4��s������H��0��
��1����ͼ��ʾװ����ȡ����������ѡ����Լ���________________________��
��2���Ʊ���������淋�װ����ͼ13��7��ʾ���Ѱ����Ͷ�����̼ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ��������С�������������Ȼ�̼�С���������϶�ʱ��ֹͣ�Ʊ���
ע�����Ȼ�̼��Һ��ʯ����Ϊ���Խ��ʡ�
�ٷ������ñ�ˮ��ȴ��ԭ����________________________________________________________________________________________________________________________________________________��
Һ��ʯ������ƿ��������________________________________________________________________________��
�ڴӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽����________________________________________________________________________
����д�������ƣ���Ϊ�˵õ������Ʒ��Ӧ��ȡ�ķ�����________����дѡ����ţ���
a����ѹ���Ⱥ��
b����ѹ���Ⱥ��
c�����40 �����º��
��β������װ����ͼ��ʾ��
˫ͨ�����ܵ����ã�____________��Ũ��������ã�______________________��__________________________________________________________________��
��3��ȡ�ֱ��ʶ�����̼����淋İ����������Ʒ1.173 0 g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ�����������Ϊ1.500 g������Ʒ�а�������淋����ʵ�������Ϊ________��[Mr��NH2COONH4����78��Mr��NH4HCO3����79��Mr��CaCO3����100]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����л�����ϩ������ȥ��ϩ�õ������ļ��飬�������ͨ��ʢ��������Щ�Լ���ϴ��ƿ�� ��
| A������ʯ��ˮ��ŨH2SO4 | B������KMnO4��ŨH2SO4 |
| C����ˮ��ŨH2SO4 | D��ŨH2SO4����ˮ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com