
����Ŀ��ij��ѧ����С��ʵ������ȡ��������ʱ�����������£�CH3COOH+C2H5OH![]() CH3COOC2H5+H2O�����ݲ�õ������������ͼ��ʾ��װ��(�г�װ�ú���)��ȡ�����������������������£�
CH3COOC2H5+H2O�����ݲ�õ������������ͼ��ʾ��װ��(�г�װ�ú���)��ȡ�����������������������£�
������ͼ��������ƿ�м���3mL�Ҵ�����ҡ������������3mLŨ���ᣬ�ڷ�Һ©����װ��3��2���Ҵ���������Һ��
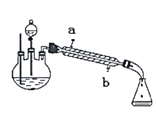
����ԡ����������ƿ��һ���¶ȣ�Ȼ��ѷ�Һ©���еĻ��Һ�����ص���������ƿ�ﲢ���ַ�Ӧ�������һ���¶ȡ�
�۷�Ӧһ��ʱ�������ƿ�л������뱥��Na2CO3��Һ��������ҡ�����ֲ����з�Һ��
���ñ���ʳ��ˮ���Ȼ�����Һϴ�����㣬�ٷ�Һ�������������������ô�����������
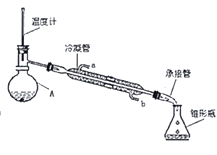
�ݽ�����������ת����ͼ������A�У���ˮԡ�м��ȣ��ռ�74��80�����ּ��ô�����ˮ����ζ��ɫ��Һ�塣
������ĿҪ��ش�
��1����ʵ����Ũ���������_____________��
��2���i�������ԡ���ȱ���120-125���¶ȷ�Χ������¶���170�棬����һ������Ӧ����д���÷�Ӧ�Ļ�ѧ����ʽ_______
��3��ͼ2������A��������____________������������ˮ��______(��a��b)�ڽ��롣
��4������ۺ͢��ж��õ��˷�Һ�������ò����õ�����Ҫ����������_________���ڷ�Һ����ʱ����������Һ���Ƴ��ķ�����_______��
��5��������и���������������ѡ�õĸ����Ϊ______(����ĸ)��
a������������ b����ˮNa2SO4 c����ʯ��
���𰸡���������ˮ�� CH3CH2OH![]() CH2=CH2��+H2O ������ƿ b ��Һ©�� ��Һ©�����²�Һ����¿��������ϲ�Һ����Ͽڵ��� b
CH2=CH2��+H2O ������ƿ b ��Һ©�� ��Һ©�����²�Һ����¿��������ϲ�Һ����Ͽڵ��� b
��������
��1��������Ӧ�ǿ��淴Ӧ����Ӧ����ˮ���ɣ�����ʵ����Ũ����������Ǵ�������ˮ�����ʴ�Ϊ����������ˮ����
��2������¶���170�棬����һ������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH![]() CH2=CH2��+H2O���ʴ�Ϊ��CH3CH2OH
CH2=CH2��+H2O���ʴ�Ϊ��CH3CH2OH![]() CH2=CH2��+H2O��
CH2=CH2��+H2O��
��3�����������Ľṹ�ص��֪ͼ2������A��������������ƿ������������ˮ�������ķ����෴������ˮ��b�ڽ����ʴ�Ϊ��b��
��4������ۺ͢��ж��õ��˷�Һ�������ò����õ�����Ҫ���������Ƿ�Һ©�����ڷ�Һ����ʱ��Ϊ��ֹ�Լ������Ⱦ������������Һ���Ƴ��ķ����Ƿ�Һ©�����²�Һ����¿��������ϲ�Һ����Ͽڵ������ʴ�Ϊ����Һ©�����²�Һ����¿��������ϲ�Һ����Ͽڵ�����
��5�������������������Ի������Һ��������ˮ�ⷴӦ��������и�����������ʱ����ѡ�����Ի���Ը���������������������Ը��������ʯ��Ϊ���Ը��������ѡb��
�ʴ�Ϊ��b��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(FeC2O4)����������������Ӱ���Լ����͵�ز�����������﮵�������ij����������Ʒ(�����ᾧˮ)�к����������ᣬ���õζ����ⶨ����Ʒ��FeC2O4�ĺ�����ʵ�鷽�����£�
�ٽ�0.20 g����������Ʒ����250 mL��ƿ�ڣ���������2 mol��L��1��H2SO4��Һ��ʹ��Ʒ�ܽ⣬������70 �����ң������ø��������Һ�ζ����յ㡣
����ζ��յ���Һ�м���������Zn�ۺ�����2 mol��L��1��H2SO4��Һ�����5��8 min����KSCN��Һ�ڵ�ΰ��ϼ������Һ��ֱ����Һ����죬�����������һ����ƿ�У���0.020 00 mol��L��1�ĸ�����ر���Һ�ζ�����Һ���յ㣬���ĸ�����ر�Һ6.00 mL��
�Իش��������⣺
(1)������ر�Һ��________�ζ���ʢװ(������ʽ��������ʽ��)��
(2)�ڲ�����У��μӸ��������Һʱ�۲쵽����ɫ���������������������ᷴӦ�����ӷ���ʽΪ______________________________________________________��
(3)�ζ��������۾�Ӧע��__________________ ���ζ��յ������� ___________________
(4)�ڲ�����У����в���������ⶨ���ƫ�ߵ���_____________��
a �ζ�����ʢװ�������ǰδ��ϴ
b �ζ������У���ƿ��̫���ң����²���Һ�彦��
c �ζ�ǰ������ȷ���ζ��յ�ʱ���Ӷ���
d �ζ�ǰ���������ݣ��ζ���������ʧ
(5)0.20 g��Ʒ��FeC2O4����������Ϊ____��(����3λ��Ч���֣������Dz�����е����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ�����A��B�����2L���ܱ������У��������·�Ӧ��3A(g)+B(g)![]() xC(g)+2D(g)����5min���D��Ũ��Ϊ0.5mol/L��C��ƽ����Ӧ������0.1mol/(L��min)���Ҵ�ʱA�����ʵ���Ũ��Ϊ0.25 mol/L��
xC(g)+2D(g)����5min���D��Ũ��Ϊ0.5mol/L��C��ƽ����Ӧ������0.1mol/(L��min)���Ҵ�ʱA�����ʵ���Ũ��Ϊ0.25 mol/L��
(1)x��ֵ��__________��
(2)B��ƽ����Ӧ����__________��
(3)��Ӧ��ʼǰ����������A�����ʵ���_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������п��a��b���ֱ���������ϡ�����У�a��ͬʱ��������CuSO4��Һ������ͼ�б�ʾ�����H2�������(V)��ʱ��(t)�Ĺ�ϵ��ȷ���ǣ� ��
A.  B.
B. 
C.  D.
D. 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ������(N2H4)Ϊȼ�ϵ����ͻ�����صĹ���ԭ����ͼ��ʾ������˵����ȷ����
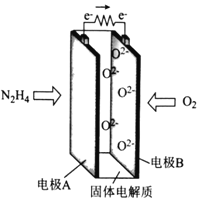
A. �缫A�ĵ��Ʊȵ缫B�ĵ�
B. �缫A�ĵ缫��ӦʽΪN2H4��4e��+4OH��===N2+4H2O
C. �缫B����������Ӧ
D. ÿ����11.2L��O2��ת�Ƶĵ�����Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о���ѧ��Ӧ�����仯����ѧ��Ӧ���ʺͻ�ѧ��Ӧ�ȣ���ʵ�ʹ�ҵ������������������Ҫ�����塣�ش��������⣺
��1����ѧ�����������FeO���պ�����CO2����ԭ��Ϊ��
��֪��C(s)+2H2O(g)====CO2(g)+2H2(g) ��H=+113.4kJ��mol��1
3FeO(s)+H2O(g)====Fe3 O4(s)+H2(g) ��H=+18.7kJ��mol��1
��Ӧ6FeO(s)+CO2(g)====2Fe3O4(s)+C(s)�ġ�H=_______��
��2���µ��Ʊ��������ô����������������İ���
��֪ClO��ˮ��ķ���ʽΪ��ClO��+H2O![]() HClO+OH���������£���ˮ�ⷴӦ��ƽ�ⳣ��ΪKh=1.0��10��6mol��L��1����1.0mol��L��1NaClO��Һ��pH=______��
HClO+OH���������£���ˮ�ⷴӦ��ƽ�ⳣ��ΪKh=1.0��10��6mol��L��1����1.0mol��L��1NaClO��Һ��pH=______��
��3����ҵ�����ð�������������(HCN)�ķ�ӦΪ��CH4(g)+NH3(g) ![]() HCN(g)+3H2 (g) ��H>0
HCN(g)+3H2 (g) ��H>0
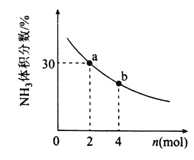
����������һ�����ﵽƽ��ʱNH3ת�������������X�仯�Ĺ�ϵ����ͼ��ʾ��X��������______(��¶ȡ���ѹǿ��)��
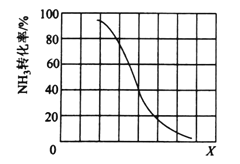
����������һ������2L�ܱ������м���nmolCH4��2molNH3��ƽ��ʱNH3���������n�仯�Ĺ�ϵ����ͼ��ʾ������Ӧ�ӿ�ʼ��a������ʱ��Ϊ10min����ʱ�������CH4��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ______�����¶��£�b���ƽ�ⳣ��Ϊ________
��4������������ͭCu2O��һ����;�㷺�Ĺ����ϣ��绯ѧ������ͭ����ʯī���缫�����Cu(NO3)2ϡ��Һ�Ʊ������������������������ͭ����______������������Cu2O�ĵ缫��ӦʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ܶ࣬����H2SO4��H2SO3��SO2��Na2SO3��BaSO4��CuSO4��Na2SO4��7�ֳ����ĺ�����ش��������⣺
(1)H2SO3ת��Ϊ�����������γɵ���Ҫ����֮һ��д���䷴Ӧ�Ļ�ѧ����ʽ������������ת�Ʒ������Ŀ��_________________________________��
(2)Ba2+�о綾��ij��������һ�������ձ����¼����������ձ�̯���������ձ��Ĺ�������̼�ᱵ�����ɷ�ʹ�ã����¶���ʳ���ձ����ж�����д��̼�ᱵ��θ��(�������ʾ)��Ӧ�����ӷ�Ӧ����ʽ______________________________��
(3)�����£�����������Ũ�����У����������α��Ͻ���Ϊ�����˶ۻ�����������Ϊδ������Ӧ��Ϊ��֤�˹��̣�ijͬѧ����˼�������������ʵ�飺����Ũ���ᴦ����������ϴ��������CuSO4��Һ�У�����������____________�������˶ۻ�������������________________����δ������Ӧ��
(4)��Na2SO3���շ���Ϊ����SO2��Ⱦ��һ�ַ�������ԭ��Ϊ(�û�ѧ����ʽ��ʾ)��______________________________��
(5)����SO2����Ⱦ�����Ϊ�����ҹ�����̽����һ����������CO��ԭSO2�õ�������ķ�������ȥSO2���÷�Ӧ�Ļ�ѧ����ʽ��____________________________��
(6)��ȡ����ͭ�����ַ���������һ��2Cu+O2![]() 2CuO��CuO+ H2SO4�� CuSO4+H2O��
2CuO��CuO+ H2SO4�� CuSO4+H2O��
��������Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O������һ�뷽������ȣ����ŵ��ǣ�_____________________________________________(����һ��)��
CuSO4+SO2��+2H2O������һ�뷽������ȣ����ŵ��ǣ�_____________________________________________(����һ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ���������ֵ��������������ȷ����
A.���³�ѹ�£�48gO2��O3�Ļ�����к��е���ԭ����Ϊ3NA
B.0.1 mol��L��1 NaCl��Һ��Na+����ΪNA
C.4��ʱ9mLˮ�ͱ�״����11.2L����������ͬ��ԭ����
D.ͬ��ͬѹ�£�NA��NO��NA��N2��O2�Ļ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ�������������ڱ���ѧϰԪ�ػ�����֪ʶ����Ҫ���ߡ�C��Si��N��P�ֱ�λ��ͬһ���塣����̬�⻯��ķֽ��¶����±���
���� | CH4 | SiH4 | NH3 | PH3 |
�ֽ��¶�/K | 873 | 773 | 1073 | T |
��1��CH4�ֽ��¶ȸ���SiH4��ԭ����_________��
��2��������Ԫ�طǽ�����ǿ����ϵ��Ԥ��PH3�ֽ��¶ȣ�T���ķ�Χ________��
��3��ijͬѧΪ֤���ǽ�����![]() �������ͼװ�ã�
�������ͼװ�ã�
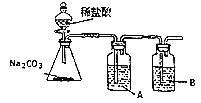
��A��ʢ�ŵ��Լ���__________��
��B�г��ֵ�������______��B�����������ӷ�Ӧ����ʽΪ_____________��
�۸�ͬѧ������д��ڵ�������__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com