
ΓΨΧβΡΩΓΩΑΔΥΨΤΞΝ÷Θ®Μ·ΚœΈοLΘ© «»ΥΟ« λ÷ΣΒΡΫβ»»’ρΆ¥“©ΈοΓΘ“Μ÷÷≥Λ–ßΓΔΜΚ ΆΑΔΥΨΤΞΝ÷Θ®Μ·ΚœΈοPΘ©ΒΡΚœ≥…¬ΖœΏ»γœ¬ΆΦΥυ ΨΘΚ
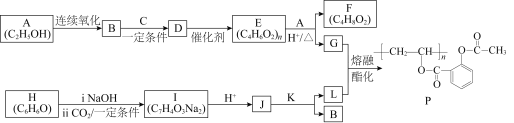
“―÷ΣΘΚΔΌHCΓ‘CH+RCOOH![]()
![]()
ΔΎRCOORΓ·+RΓ±OH![]() RCOORΓ±+RΓ·OHΘ®RΓΔRΓ·ΓΔRΓ±¥ζ±μΧΰΜυΘ©
RCOORΓ±+RΓ·OHΘ®RΓΔRΓ·ΓΔRΓ±¥ζ±μΧΰΜυΘ©
«κΜΊ¥πΘΚ
(1)A÷–ΒΡΙΌΡήΆ≈ «____________________ΓΘ
(2)CΒΡΫαΙΙΦρ Ϋ «____________________ΓΘ
(3)DΓζEΒΡΖ¥”Πάύ–Ά «____________________ΓΘ
(4)EΓζGΒΡΜ·―ßΖΫ≥Χ Ϋ «______________________________________ΓΘ
(5)“―÷ΣΘΚH «ΖΦœψΉεΜ·ΚœΈοΓΘ‘Ύ“ΜΕ®ΧθΦΰœ¬2B Γζ K + H2OΘ§KΒΡΚΥ¥≈Ι≤’ώ«βΤΉ÷Μ”–“ΜΉιΖεΓΘJΓζLΒΡΜ·―ßΖΫ≥Χ Ϋ «____________________ΓΘ
(6)L‘ΎΧεΡΎΩ…ΫœΩλΉΣΜ·ΈΣΨΏ”–“©–ßΒΡJΘ§ΕχΜ·ΚœΈοP”κLœύ±»Θ§‘ΎΧεΡΎΡήΜΚ¬ΐ≥÷–χ ΆΖ≈JΓΘ
ΔΌ ―Σ“Κ÷–J≈®Ε»ΙΐΗΏΡή Ι»Υ÷–ΕΨΘ§Ω…Ψ≤¬ωΒΈΉΔNaHCO3»ή“ΚΫβΕΨΓΘ«κ”ΟΜ·―ßΖΫ≥Χ ΫΫβ ΆNaHCO3ΒΡΉς”ΟΘΚ______________________________________________________________ΓΘ
ΔΎ œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «______Θ®ΧνΉ÷ΡΗΘ©ΓΘ
aΘ°P÷–ΒΡθΞΜυ‘ΎΧεΡΎΩ…ΜΚ¬ΐΥ°ΫβΘ§÷πΫΞ ΆΖ≈≥ωJ
bΘ°P‘ΎΧεΡΎΒΡΥ°Ϋβ≤ζΈο÷–ΟΜ”–ΗΏΖ÷Ή”Μ·ΚœΈο
cΘ°ΫΪ–ΓΖ÷Ή”“©Έο“ΐ»κΒΫΗΏΖ÷Ή”÷–Ω…“‘ Βœ÷“©ΈοΒΡΜΚ ΆΙΠΡή
ΓΨ¥πΑΗΓΩτ«Μυ HCΓ‘CH Φ”ΨέΖ¥”Π 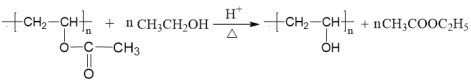

![]() ac
ac
ΓΨΫβΈωΓΩ
A «![]() Θ§““¥ΦΝ§–χ―θΜ·ΒΟ““ΥαΘ§Ι B «
Θ§““¥ΦΝ§–χ―θΜ·ΒΟ““ΥαΘ§Ι B «![]() Θ§ΗυΨί–≈œΔΔΌΘ§ΫαΚœΝς≥ΧΆΦΘ§C «
Θ§ΗυΨί–≈œΔΔΌΘ§ΫαΚœΝς≥ΧΆΦΘ§C «![]() Θ§D «
Θ§D «![]() Θ§DΒΫE «Φ”ΨέΖ¥”ΠΘ§E «
Θ§DΒΫE «Φ”ΨέΖ¥”ΠΘ§E « Θ§ΗυΨί–≈œΔΔΎΩ…ΆΤ÷ΣFΈΣ
Θ§ΗυΨί–≈œΔΔΎΩ…ΆΤ÷ΣFΈΣ![]() Θ§G «
Θ§G «![]() Θ§ΗυΨίΖ÷Ή” ΫH «
Θ§ΗυΨίΖ÷Ή” ΫH «![]() Θ§ΗυΨίΝς≥ΧΆΦΫαΚœPΒΡΫαΙΙΩ…ΆΤ÷ΣIΈΣ
Θ§ΗυΨίΝς≥ΧΆΦΫαΚœPΒΡΫαΙΙΩ…ΆΤ÷ΣIΈΣ Θ§J «
Θ§J «![]() Θ§ΗυΨίΘ®5Θ©–ΓΧβΘ§Ω…“‘ΆΤ÷ΣK «““ΥατϊΘ®
Θ§ΗυΨίΘ®5Θ©–ΓΧβΘ§Ω…“‘ΆΤ÷ΣK «““ΥατϊΘ®![]() Θ©Θ§”…PΡφΆΤ÷ΣLΈΣ
Θ©Θ§”…PΡφΆΤ÷ΣLΈΣ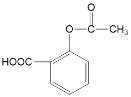 ΓΘ
ΓΘ
Θ®1Θ©A «““¥ΦΘ§““¥ΦΒΡΙΌΡήΆ≈ «τ«ΜυΘ§
¥πΑΗΈΣΘΚτ«ΜυΘΜ
Θ®2Θ©Ψί–≈œΔΔΌΘ§ΫαΚœΝς≥ΧΆΦΘ§Ω…“‘ΆΤ≥ωC «![]() Θ§
Θ§
Ι ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
Θ®3Θ©D «![]() Θ§ E «
Θ§ E « Θ§DΒΫE «ΥΪΦϋΖΔ…ζΝΥΦ”ΨέΖ¥”ΠΘ§
Θ§DΒΫE «ΥΪΦϋΖΔ…ζΝΥΦ”ΨέΖ¥”ΠΘ§
Ι ¥πΑΗΈΣΘΚΦ”ΨέΖ¥”ΠΘΜ
Θ®4Θ©E « Θ§A «““¥ΦΘ§ΗυΨί–≈œΔΔΎΘ§ΗΟΖ¥”ΠΈΣ»Γ¥ζΖ¥”ΠΘ§
Θ§A «““¥ΦΘ§ΗυΨί–≈œΔΔΎΘ§ΗΟΖ¥”ΠΈΣ»Γ¥ζΖ¥”ΠΘ§
¥πΑΗΈΣΘΚ ΘΜ
ΘΜ
Θ®5Θ©ΗυΨί–≈œΔΩ…ΆΤ÷ΣJ «![]() Θ§K «““ΥατϊΘ®
Θ§K «““ΥατϊΘ® Θ©Θ§
Θ©Θ§
¥πΑΗΈΣΘΚ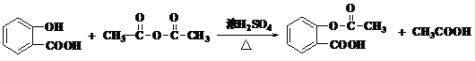 ΘΜ
ΘΜ
Θ®6Θ©ΔΌτ»ΥαΒΡΥα–‘¥σ”ΎΧΦΥαΘ§Ζ”ΒΡΥα–‘–Γ”ΎΧΦΥαΘ§ Ι Ζ”τ«ΜυΚΆΧΦΥα«βΡΤ≤ΜΖ¥”ΠΘ§τ»ΜυΚΆΧΦΥα«βΡΤΖ¥”Π…ζ≥…Εΰ―θΜ·ΧΦΘ§
Ι ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
ΔΎa.PΩ…“‘Άξ»ΪΥ°Ϋβ≤ζ…ζ![]() Θ§
Θ§![]() “‘ΦΑ““ΥαΘ§a’ΐ»ΖΘΜ
“‘ΦΑ““ΥαΘ§a’ΐ»ΖΘΜ
b. PΩ…“‘Άξ»ΪΥ°Ϋβ≤ζ…ζ![]() Θ§ΗΟΈο÷ ΈΣΗΏΖ÷Ή”Θ§b¥μΈσΘΜ
Θ§ΗΟΈο÷ ΈΣΗΏΖ÷Ή”Θ§b¥μΈσΘΜ
c. ΗυΨίΧβΡΩ–≈œΔΘ§ΫΪ–ΓΖ÷Ή”“©Έο“ΐ»κΒΫΗΏΖ÷Ή”÷–Ω…“‘ Βœ÷“©ΈοΒΡΜΚ ΆΙΠΡήΘ§c’ΐ»ΖΘΜ
¥πΑΗ―ΓacΓΘ


 Ή¥‘ΣΖΜ»Ϊ≥ΧΆΜΤΤΒΦΝΖ≤βœΒΝ–¥πΑΗ
Ή¥‘ΣΖΜ»Ϊ≥ΧΆΜΤΤΒΦΝΖ≤βœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ‘Ύ “Έ¬œ¬Θ§œ¬Ν–Έε÷÷»ή“ΚΘΚΔΌ0.1 mol/L NH4Cl»ή“ΚΘ§ΔΎ0.1 mol/L CH3COONH4»ή“ΚΘ§Δέ0.1 mol/L NH4HSO4»ή“ΚΘ§Δή0.1 mol/L Α±Υ°Θ§Δί0.1 mol/L NH3ΓΛH2OΚΆ0.1 mol/L NH4ClΜλΚœ“ΚΘ®œ‘Φν–‘Θ©ΓΘ«κΗυΨί“Σ«σΧν–¥œ¬Ν–Ω’ΑΉΘΚ
Θ®1Θ©»ή“ΚΔΌ≥ ____________–‘Θ®ΧνΓΑΥαΓ±ΓΔΓΑΦνΓ±ΜρΓΑ÷–Γ±Θ©Θ§Τδ‘≠“ρ «___________Θ®”ΟάκΉ”ΖΫ≥Χ Ϋ±μ ΨΘ©
Θ®2Θ©‘Ύ…œ ωΔΌΓΔΔΎΓΔΔέΓΔΔή»ή“Κ÷–cΘ®NH4+Θ©≈®Ε»”…¥σΒΫ–ΓΒΡΥ≥–ρ «_________ΓΘΘ®Χν–ρΚ≈Θ©
Θ®3Θ©‘Ύ»ή“ΚΔί÷–Θ§ΗςάκΉ”≈®Ε»¥σ–ΓΥ≥–ρΈΣ___________ΓΘ
Θ®4Θ© “Έ¬œ¬Θ§≤βΒΟ»ή“ΚΔΎΒΡpH=7Θ§‘ρ CH3COOΓΣ”κNH4+≈®Ε»ΒΡ¥σ–ΓΙΊœΒ «cΘ®CH3COOΓΣΘ©________cΘ®NH4+Θ©Θ®ΧνΓΑ>Γ±ΓΔΓΑ<Γ±ΜρΓΑ=Γ±Θ©ΓΘ
Θ®5Θ©≥ΘΈ¬œ¬Θ§0.1 mol/ L CH3COOH»ή“ΚΦ”Υ°œΓ ΆΙΐ≥Χ÷–Θ§œ¬Ν–±μ¥ο ΫΒΡ ΐ÷Β±δ¥σΒΡ «____________Θ®ΧνΉ÷ΡΗΘ©ΓΘ
A. c(H+) B. c(H+)/ c(CH3COOH) C. c(H+)ΓΛc(OHΘ≠)
D. c(OHΘ≠)/ c(H+) E. c(H+)ΓΛc(CH3COOΘ≠) / c(CH3COOH)
Θ®6Θ©25Γφ ±Θ§ΫΪpH=9ΒΡNaOH»ή“Κ”κpH=4ΒΡH2SO4»ή“ΚΜλΚœΘ§»τΥυΒΟΜλΚœ»ή“ΚΒΡpH=7Θ§‘ρNaOH»ή“Κ”κH2SO4»ή“ΚΒΡΧεΜΐ±»ΈΣ____________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩaΓΔbΓΔcΓΔd ΥΡ÷÷ΕΧ÷ήΤΎ‘ΣΥΊ‘Ύ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο»γΆΦΥυ ΨΘ§a ΚΆ b Ζ÷±πΈΜ”Ύ÷ήΤΎ±μΒΡΒΎ 2 Ν–ΚΆΒΎ 13 Ν–Θ§ œ¬Ν––π ω’ΐ»ΖΒΡ

A.άκΉ”ΑκΨΕ b>d
B.b Ω…“‘ΚΆ«ΩΦν»ή“ΚΖΔ…ζΖ¥”Π
C.c ΒΡ«βΜ·ΈοΩ’ΦδΫαΙΙΈΣ»ΐΫ«ΉΕ–Έ
D.a ΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·Έο ««ΩΦν
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ¬Χ…ΪΜ·―ß”÷≥ΤΜΖΨ≥”―ΚΟΜ·―ßΘ§ΥϋΒΡ÷ς“ΣΧΊΒψ÷°“Μ «ΧαΗΏ‘≠Ή”ΒΡάϊ”Ο¬ Θ§ Ι‘≠Νœ÷–Υυ”–ΒΡ‘≠Ή”»Ϊ≤Ω ΉΣΜ·ΒΫ≤ζΤΖ÷–Θ§ Βœ÷ΓΑΝψ≈≈Ζ≈Γ±ΓΘœ¬Ν–Ζ¥”ΠΖϊΚœ¬Χ…ΪΜ·―ß’β“ΜΧΊΒψΒΡ «
A. ΙΛ“Β“±ΝΕ Fe2O3+3CO ![]() 2Fe+3CO2
2Fe+3CO2
B. ”Ο…ζ ·Μ“÷Τ λ ·Μ“ CaO+H2O=Ca(OH)2
C. Β―ι “÷Τ»ΓΕΰ―θΜ·ΧΦ CaCO3+2HCl=CaCl2+H2O+CO2Γϋ
D. Β―ι “÷Τ»Γ«βΤχ Zn+H2SO4=ZnSO4+H2Γϋ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩAlN–¬–Ά≤ΡΝœ”Π”Ο«ΑΨΑΙψΖΚΘ§Τδ÷Τ±Η”κ–‘÷ ―–ΨΩ≥…ΈΣ»»ΒψΓΘ
œύΙΊ ΐΨί»γœ¬ΘΚ
Έο÷ | »έΒψ/Γφ | Ζ–Βψ/Γφ | ”κN2Ζ¥”ΠΈ¬Ε»/Γφ | œύ”ΠΜ·ΚœΈοΖ÷ΫβΈ¬Ε»/Γφ |
Al | 660 | 2467 | ΘΨ800 | AlNΘΚΘΨ2000 Θ®ΘΨ1400…ΐΜΣΘ© AlCl3ΘΚΘ®ΘΨ181…ΐΜΣΘ© |
Mg | 649 | 1090 | ΘΨ300 | Mg3N2ΘΚΘΨ800 |
(1)AlNΒΡ÷Τ±ΗΓΘ
ΔΌ Μ·―ßΤχœύ≥ΝΜΐΖ®ΓΘ
Δώ.“ΜΕ®Έ¬Ε»œ¬Θ§“‘AlCl3ΤχΧεΚΆNH3ΈΣ‘≠Νœ÷Τ±ΗAlNΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «____________________ΓΘ
Δρ.…œ ωΖ¥”Π “ΥΒΡΈ¬Ε»ΖΕΈß «______ΓφΘ®ΧνΉ÷ΡΗΘ©ΓΘ
a.75~100 b.600~1100 c.2000~2300
ΔΎ ¬ΝΖέ÷±Ϋ”ΒΣΜ·Ζ®ΓΘ
Al”κN2Ω…÷±Ϋ”Μ·ΚœΈΣAlNΙΧΧεΘ§AlNΡήΫΪAlΑϋΙϋΘ§Ζ¥”ΠΡ―“‘ΦΧ–χΫχ––ΓΘΩΊ÷ΤΈ¬Ε»Θ§‘ΎAlΖέ÷–Ψυ‘»≤τ»κ ΝΩMgΖέΘ§Ω… ΙAlΦΗΚθ»Ϊ≤ΩΉΣΜ·ΈΣAlNΙΧΧεΓΘΗΟΙΐ≥ΧΖΔ…ζΒΡΖ¥”Π”–ΘΚ__________________ΓΔ_________ΚΆ2Al + N2 ![]() 2AlNΓΘ
2AlNΓΘ
ΔέΧΦ»»ΜΙ‘≠Ζ®ΓΘ
“‘Al2O3ΓΔCΘ® ·ΡΪΘ©ΚΆN2ΈΣ‘≠ΝœΘ§‘ΎΗΏΈ¬œ¬÷Τ±ΗAlNΓΘ
“―÷ΣΘΚΔΓ. 2Al2O3(s) 4Al(g) + 3O2(g) H 1 =ΘΪ3351 kJΓΛmol-1
ΔΔ. 2C( ·ΡΪΘ§s) + O2(g) = 2CO(g) H 2 =Θ≠221 kJΓΛmol-1
ΔΘ. 2Al(g) + N2(g) = 2AlN(s) H 3 =Θ≠318 kJΓΛmol-1
‘Υ”ΟΤΫΚβ“ΤΕ·‘≠άμΖ÷ΈωΖ¥”ΠΔΔΕ‘Ζ¥”ΠΔΓΒΡΩ…Ρή”ΑœλΘΚ______________________________________ΓΘ
(2)AlNΒΡ–‘÷ ΓΘAlNΖέΡ©Ω…ΖΔ…ζΥ°ΫβΓΘœύΆ§ΧθΦΰœ¬Θ§≤ΜΆ§ΝΘΨΕΒΡAlNΖέΡ©Υ°Ϋβ ±»ή“ΚpHΒΡ±δΜ·»γΆΦΥυ ΨΓΘ
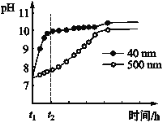
ΔΌ AlNΖέΡ©Υ°ΫβΒΡΜ·―ßΖΫ≥Χ Ϋ «____________________________________ΓΘ
ΔΎ Ϋβ Άt1-t2 ±ΦδΡΎΝΫΧθ«ζœΏ≤ν“λΒΡΩ…Ρή‘≠“ρΘΚ_______________________________ΓΘ
(3)AlNΚ§ΝΩΦλ≤βΓΘœρa g AlN―υΤΖ÷–Φ”»κΉψΝΩ≈®NaOH»ή“ΚΘ§»ΜΚσΆ®»κΥ°’τΤχΫΪNH3»Ϊ≤Ω’τ≥ωΘ§ΫΪNH3”ΟΙΐΝΩΒΡv1 mL c1 molΓΛL-1 H2SO4»ή“ΚΈϋ ’Άξ»ΪΘ§ Θ”ύΒΡH2SO4”Οv2 mL c2 molΓΛL-1 NaOH»ή“Κ«ΓΚΟ÷–ΚΆΘ§‘ρ―υΤΖ÷–AlNΒΡ÷ ΝΩΖ÷ ΐ «________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩCoCl2Ω…”Ο”ΎΒγΕΤΘ§ «“Μ÷÷–‘Ρή”≈‘ΫΒΡΒγ≥Ί«Α«ΐ≤ΡΝœΘ§”…Κ§νήΩσ(Co‘ΣΥΊ÷ς“Σ“‘Co2O3ΓΔCoO¥φ‘ΎΘ§ΜΙΚ§”–FeΓΔSiΓΔCuΓΔZnΓΔMnΓΔNiΓΔMgΓΔCa‘ΣΥΊ)÷Τ»Γ¬»Μ·νήΨßΧεΒΡ“Μ÷÷ΙΛ“’Νς≥Χ»γœ¬ΘΚ
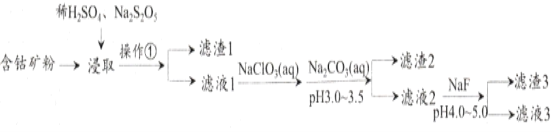
“―÷ΣΘΚΔΌΫΙ―«ΝρΥαΡΤNa2S2O5Θ§≥ΘΉω ≥ΤΖΩΙ―θΜ·ΦΝΓΘCaF2ΓΔMgF2Ρ―»ή”ΎΥ°ΓΘ
ΔΎCoCl2ΓΛ6H2O»έΒψ86ΓφΘ§“Ή»ή”ΎΥ°ΓΔ““Ο―Β»ΘΜ≥ΘΈ¬œ¬Έ»Ε®ΈόΕΨΘ§Φ”»»÷Ν110ΓΣ120Γφ ±Θ§ ß»ΞΫαΨßΥ°±δ≥…”–ΕΨΒΡΈόΥ°¬»Μ·νήΓΘ
Δέ≤ΩΖ÷Ϋπ τάκΉ”–Έ≥…«β―θΜ·ΈοΒΡpHΦϊœ¬±μΘΚ
Co3+ | Fe3+ | Cu2+ | Co2+ | Fe2+ | Zn2+ | Mn2+ | Mg2+ | |
ΩΣ Φ≥ΝΒμpH | 0.3 | 2.7 | 5.5 | 7.2 | 7.6 | 7.6 | 8.3 | 9.6 |
Άξ»Ϊ≥ΝΒμpH | 1.1 | 3.2 | 6.6 | 9.2 | 9.6 | 9.2 | 9.3 | 11.1 |
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©≤ΌΉςΔΌΒΡΟϊ≥ΤΈΣ_________Θ§NaClO3ΨΏ”–―θΜ·–‘Θ§ΤδΟϊ≥ΤΈΣ__________________ΓΘ
Θ®2Θ©Ϋΰ»Γ÷–Φ”»κNa2S2O5ΒΡΉς”Ο «___________________________ΓΘ
Θ®3Θ©¬Υ“Κ1÷–Φ”»κNaClO3/span>ΒΡΉς”Ο «_______________________________________Θ§œύΙΊΒΡάκΉ”ΖΫ≥Χ ΫΈΣ__________________________________________ΓΘ
Θ®4Θ©Φ”»κNa2CO3»ή“Κ…ζ≥…¬Υ‘ϋ2ΒΡ÷ς“ΣάκΉ”ΖΫ≥Χ ΫΈΣ___________________________ΓΘ
Θ®5Θ©¬Υ‘ϋ3÷ς“Σ≥…Ζ÷ΈΣ________________________(–¥Μ·―ß Ϋ)ΓΘ
¬Υ“Κ3Ψ≠ΙΐΕύ¥ΈίΆ»Γ”κΖ¥ίΆ»Γ÷Τ±ΗCoCl2ΨßΧε
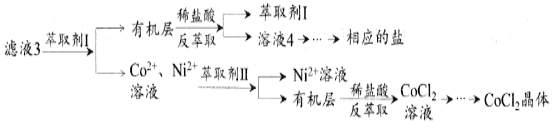
Θ®6Θ©¬Υ“Κ3÷–Φ”»κίΆ»ΓΦΝIΘ§»ΜΚσ”ΟœΓ―ΈΥαΖ¥ίΆ»ΓΒΡΡΩΒΡ «_______________________ΓΘ
Θ®7Θ©÷Τ±ΗΨßΧεCoCl2ΓΛ6H2OΘ§–η‘ΎΦθ―ΙΜΖΨ≥œ¬ΚφΗ…ΒΡ‘≠“ρ «_________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ![]() “‘
“‘![]() ΚΆ
ΚΆ![]() ΈΣ‘≠ΝœΚœ≥…ΡρΥΊ «άϊ”Ο
ΈΣ‘≠ΝœΚœ≥…ΡρΥΊ «άϊ”Ο![]() ΒΡ≥…ΙΠΖΕάΐΓΘ‘ΎΡρΥΊΚœ≥…Υΰ÷–ΒΡ÷ς“ΣΖ¥”ΠΩ…±μ Ψ»γœ¬ΘΚ
ΒΡ≥…ΙΠΖΕάΐΓΘ‘ΎΡρΥΊΚœ≥…Υΰ÷–ΒΡ÷ς“ΣΖ¥”ΠΩ…±μ Ψ»γœ¬ΘΚ
Ζ¥”ΠΔώΘΚ![]()
![]()
Ζ¥”ΠΔρΘΚ![]()
![]()
ΉήΖ¥”ΠΘΚ![]()
![]()
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
![]() Ζ¥”ΠΔώΒΡ
Ζ¥”ΠΔώΒΡ![]() ______________ΓΘ
______________ΓΘ
![]() ‘Ύ____
‘Ύ____![]() ΧνΓΑΗΏΈ¬Γ±ΜρΓΑΒΆΈ¬Γ±
ΧνΓΑΗΏΈ¬Γ±ΜρΓΑΒΆΈ¬Γ±![]() «ιΩωœ¬”–άϊ”ΎΖ¥”ΠΔρΒΡΉ‘ΖΔΫχ––ΓΘ
«ιΩωœ¬”–άϊ”ΎΖ¥”ΠΔρΒΡΉ‘ΖΔΫχ––ΓΘ
![]() Α±Ζ®»ήΫΰ―θΜ·–Ω―ΧΜ“÷Τ»ΓΗΏ¥Ω–ΩΒΡΙΛ“’Νς≥Χ»γΆΦΥυ ΨΓΘ»ήΫΰΚσ―θΜ·–Ω―ΧΜ“÷––ΩΓΔΆ≠ΓΔο”ΓΔ…ι‘ΣΥΊΖ÷±π“‘
Α±Ζ®»ήΫΰ―θΜ·–Ω―ΧΜ“÷Τ»ΓΗΏ¥Ω–ΩΒΡΙΛ“’Νς≥Χ»γΆΦΥυ ΨΓΘ»ήΫΰΚσ―θΜ·–Ω―ΧΜ“÷––ΩΓΔΆ≠ΓΔο”ΓΔ…ι‘ΣΥΊΖ÷±π“‘![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() ΒΡ–Έ Ϋ¥φ‘ΎΓΘ
ΒΡ–Έ Ϋ¥φ‘ΎΓΘ

![]() ΓΑ»ήΫΰΓ±÷– ZnOΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ _______ΓΘ
ΓΑ»ήΫΰΓ±÷– ZnOΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ _______ΓΘ
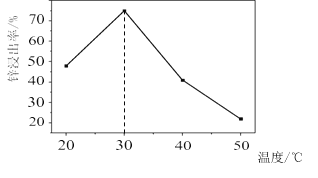
![]() –ΩΫΰ≥ω¬ ”κΈ¬Ε»ΒΡΙΊœΒ»γΆΦΥυ ΨΘ§Ζ÷Έω
–ΩΫΰ≥ω¬ ”κΈ¬Ε»ΒΡΙΊœΒ»γΆΦΥυ ΨΘ§Ζ÷Έω ![]() ±–ΩΫΰ≥ω¬ ΉνΗΏΒΡ‘≠“ρΈΣ __________ΓΘ
±–ΩΫΰ≥ω¬ ΉνΗΏΒΡ‘≠“ρΈΣ __________ΓΘ
![]() ΓΑ¬Υ‘ϋ 3Γ±ΒΡ÷ς“Σ≥…Ζ÷ΈΣ_______________ΓΘ
ΓΑ¬Υ‘ϋ 3Γ±ΒΡ÷ς“Σ≥…Ζ÷ΈΣ_______________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ÷–ΚΆ»»ΒΡ≤βΕ® «ΗΏ÷–÷Ί“ΣΒΡΕ®ΝΩ Β―ιΓΘ»Γ0.55mol/LΒΡNaOH»ή“Κ50mL”κ0.25mol/LΒΡΝρΥα50mL÷Ο”Ύœ¬ΆΦΥυ ΨΒΡΉΑ÷Ο÷–Ϋχ––÷–ΚΆ»»ΒΡ≤βΕ® Β―ιΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ

Θ®1Θ©¥”…œΆΦ Β―ιΉΑ÷ΟΩ¥Θ§Τδ÷–…–»±…ΌΒΡ“Μ÷÷≤ΘΝß”ΟΤΖ «__________Θ§≥ΐ¥Υ÷°ΆβΘ§ΉΑ÷Ο÷–ΒΡ“ΜΗωΟςœ‘¥μΈσ «___________________________________ΓΘ
Θ®2Θ©ΈΣ±Θ÷ΛΗΟ Β―ι≥…ΙΠΗΟΆ§―ß≤…»ΓΝΥ–μΕύ¥κ ©Θ§»γΆΦΒΡΥι÷ΫΧθΒΡΉς”Ο‘Ύ”Ύ_________ΓΘ
Θ®3Θ©»τΗΡ”Ο60mL 0.25molΓΛL-1 H2SO4ΚΆ50mL 0.55molΓΛL-1 NaOH»ή“ΚΫχ––Ζ¥”Π”κ…œ ω Β―ιœύ±»Θ§ΥυΖ≈≥ωΒΡ»»ΝΩ_______Θ®ΧνΓΑœύȖΓΑ≤Μœύ»ñȩȧ»τ Β―ι≤ΌΉςΨυ’ΐ»ΖΘ§‘ρΥυ«σ÷–ΚΆ»»__________Θ®ΧνΓΑœύȖΓΑ≤Μœύ»ñȩ
Θ®4Θ©ΒΙ»κNaOH»ή“ΚΒΡ’ΐ»Ζ≤ΌΉς «ΘΚ________ΓΘΘ®¥”œ¬Ν–―Γ≥ωΘ©
AΘ°―Ί≤ΘΝßΑτΜΚ¬ΐΒΙ»κ BΘ°Ζ÷»ΐ¥Έ…ΌΝΩΒΙ»κ CΘ°“Μ¥Έ―ΗΥΌΒΙ»κ
Θ®5Θ© Β―ι ΐΨί»γœ¬±μΘΚΔΌ«κΧν–¥œ¬±μ÷–ΒΡΩ’ΑΉΘΚ
Έ¬Ε» Β―ι¥Έ ΐ | Τπ ΦΈ¬Ε»t1Γφ | ÷’÷ΙΈ¬Ε»t2/Γφ | Έ¬Ε»≤νΤΫΨυ÷Β (t2Θ≠t1)/Γφ | ||
H2SO4 | NaOH | ΤΫΨυ÷Β | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | ___________ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | ||
ΔΎΫϋΥΤ»œΈΣ0.55 mol/L NaOH»ή“ΚΚΆ0.25 mol/LΝρΥα»ή“ΚΒΡΟήΕ»ΕΦ «1 g/cm3Θ§÷–ΚΆΚσ…ζ≥…»ή“ΚΒΡ±»»»»ίcΘΫ4.18 J/(gΓΛΓφ)ΓΘ‘ρ÷–ΚΆ»»ΠΛHΘΫ_________ΓΘ(Χα ΨΘΚΠΛH=Θ≠![]() ,±ΘΝτ“ΜΈΜ–Γ ΐ)ΓΘ
,±ΘΝτ“ΜΈΜ–Γ ΐ)ΓΘ
Δέ…œ ω Β―ι ΐ÷ΒΫαΙϊ”κ57.3 kJ/mol”–ΤΪ≤νΘ§≤ζ…ζΤΪ≤νΒΡ‘≠“ρΩ…Ρή «(ΧνΉ÷ΡΗ)________ΓΘ
aΘ° Β―ιΉΑ÷Ο±ΘΈ¬ΓΔΗτ»»–ßΙϊ≤ν
bΘ°”ΟΈ¬Ε»ΦΤ≤βΕ®NaOH»ή“ΚΤπ ΦΈ¬Ε»Κσ÷±Ϋ”≤βΕ®H2SO4»ή“ΚΒΡΈ¬Ε»
cΘ°Ζ÷Εύ¥ΈΑ―NaOH»ή“ΚΒΙ»κ Δ”–ΝρΥαΒΡ–Γ…’±≠÷–
Θ®6Θ©»γΙϊ”ΟΚ§0.5mol Ba(OH)2ΒΡœΓ»ή“Κ”κΉψΝΩœΓΝρΥα»ή“ΚΖ¥”ΠΘ§Ζ¥”ΠΖ≈≥ωΒΡ»»____57.3 kJΘ®ΧνΓΑ¥σ”ΎΓ±ΓΔΓΑ–Γ”ΎΓ±ΜρΓΑΒ»”ΎΓ±Θ©ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΈεΥ°Νρ¥ζΝρΥαΡΤ(Μ·―ß ΫΈΣNa2S2O3 5H20)≤Μ»ή”Ύ““¥ΦΘ§“Ή»ή”ΎΥ°Θ§Ω…”Ο”ΎςΖ÷ΤΤΛΗοΓΔΩσ ·Χα“χΓΔΥ°≤ζ―χ÷≥ΓΔ“ΫΝΤΫβΕΨΒ»Θ§ΙΛ“Β…œ≥Θ”ΟNa2SΓΔNa2CO3ΈΣ‘≠Νœ÷Τ±ΗΘ§Τδ…ζ≤ζΝς≥Χ»γœ¬ΆΦΥυ ΨΘ§ΜΊ¥πœ¬Ν–Έ ΧβΓΘ

(1)ΈεΥ°Νρ¥ζΝρΥαΡΤ÷–Νρ‘ΣΥΊΒΡΜ·ΚœΦέΈΣ__________ΓΘ≥ΘΈ¬œ¬Θ§Na2S»ή“ΚΒΡpH_______7(―ΓΧνΓΑΘΨΓ±ΓΔΓΑΘΦΓ±ΜρΓΑ=Γ±)ΓΘ
(2)»ήΫβ‘≠ΝœΒΡ’τΝσΥ°–ηΦ”»»÷σΖ–“ΜΕΈ ±ΦδΚσ¥ΐ”ΟΘ§ΤδΡΩΒΡ «____________ΓΘ
(3)Na2SΚΆNa2CO3Α¥Έο÷ ΒΡΝΩ±»2:1ΆΕΝœΚσΦ”»»Θ§ΫΪS02ΜΚΜΚΆ®»κ»ή“Κ÷–Θ§Φ¥Ω…ΜώΒΟ Na2S203Θ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___________________________ΓΘ
(4)…œ ω÷Τ±Η Β―ι“ΜΑψ–κΩΊ÷Τ‘ΎΦν–‘ΜΖΨ≥œ¬Ϋχ––Θ§»τ‘ΎΥα–‘ΧθΦΰœ¬≤ζΤΖΜαΖΔΜΤΘ§”ΟάκΉ”ΖΫ≥Χ Ϋ±μ ΨΤδΩ…ΡήΒΡ‘≠“ρ___________________________ΓΘ
(5)≥δΖ÷Ζ¥”ΠΚσ≥Ο»»Ιΐ¬ΥΘ§ΫΪ¬Υ“ΚΥ°‘ΓΦ”»»≈®ΥθΘ§ά以Έω≥ωΚσ”Ο““¥Φœ¥Β”±μΟφΘ§Φ¥Ω…ΜώΒΟ¥ΩΨΜΒΡΈό…ΪΆΗΟςΨßΧεΓΘ≥Ο»»Ιΐ¬ΥΚΆ”Ο““¥Φœ¥Β”ΒΡΡΩΒΡ « ___________________________ΓΘ
(6)ΈΣ≤βΕ®≤ζΤΖ÷–Na2S2O3 5H2OΒΡ¥ΩΕ»Θ§ΉΦ»Ζ≥Τ»Γ―υΤΖ÷ ΝΩΘ§”Ο ΝΩΒΡ’τΝσΥ°»ήΫβΘ§Φ”»κ÷Η ΨΦΝ”Ο“―÷Σ≈®Ε»ΒΡΒβΒΡ±ξΉΦ»ή“ΚΒΈΕ®ΓΘ
Ζ¥”Π‘≠άμΈΣ:2S2O32-+I2= S4O62-+2I-
ΔΌΦ”»κΒΡ÷Η ΨΦΝΈΣ______________________(ΧνΟϊ≥Τ)ΓΘ
ΔΎΒΈΕ®÷Ν÷’Βψ ±Θ§»ή“Κ―’…ΪΒΡ±δΜ·______________________ΓΘ
ΔέΗΟ Β―ι÷–Θ§ΒΈΦ”±ξΉΦΒβ“ΚΒΡ≤ΌΉς’ΐ»ΖΒΡ «________________________________(ΧνΆΦ÷–Ε‘”Π≤ΌΉςΒΡΉ÷ΡΗ±ύΚ≈)ΓΘ

Δήœ¬Ν–≤ΌΉςΩ… Ι≤ζΤΖ¥ΩΕ»Φλ≤β÷ΒΤΪσ{ΒΡ «___________(―ΓΧν±ύΚ≈)ΓΘ
a.ΉΑ±ξΉΦ“ΚΒΡΒΈΕ®ΙήΥ°œ¥ΚσΈ¥»σœ¥
b.ΒΈΕ®Ιΐ≥Χ÷–≥ωœ÷¬©“Κ
c.ΒΈΕ®Ϋα χ ±Η© ”ΕΝ ΐ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com