
����Ŀ���ݡ���Ȼ��ͨѶ��(Nature Communications)�������ҹ���ѧ�ҷ���������ͭ���״����ڶ�����̼�绯ѧ��ԭ�������״������д�Ч�ʸߡ�ͭ������Ԫ�ػ�������������������Ӧ�ù㷺��
��ش��������⣺
(1)��̬��ԭ�ӵļ۵����Ų�ʽΪ________������������Ԫ�صļ��⻯���зе���͵���________��
(2)�绹ԭ���Ʊ��״���ԭ��Ϊ2CO2+4H2O![]() 2CH3OH+3O2��
2CH3OH+3O2��
��д���÷�Ӧ���ɼ��Լ����ɵķǼ��Է��ӵĽṹʽ________��
�ڱ�״���£�V L CO2���庬��________��������
(3)��������6��Cԭ�ӣ�ÿ��Cԭ����һ��2p��������γɴ��������ɼ�Ϊ(��![]() ���½���6����ʾ6��ԭ�ӣ����Ͻ���6����ʾ6�����õ���)����֪ij������Ľṹ��ʽΪ
���½���6����ʾ6��ԭ�ӣ����Ͻ���6����ʾ6�����õ���)����֪ij������Ľṹ��ʽΪ![]() ������ʹ������Ȼ�̼��Һ��ɫ���ɴ���֪���÷����д��ڴ��������ɱ�ʾΪ_______��Se���ӻ���ʽΪ________��
������ʹ������Ȼ�̼��Һ��ɫ���ɴ���֪���÷����д��ڴ��������ɱ�ʾΪ_______��Se���ӻ���ʽΪ________��
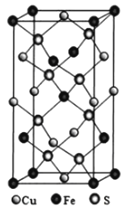
(4)��ͭ����Cu+��Fe3+��S2-���ɣ����ķ���ϵ�����ṹ��ͼ��ʾ����Cu+����λ��Ϊ________������������a=b=524pm��c=1032pm����NA��ʾ�����ӵ�������ֵ���þ�ϵ������ܶ���________g��cm-3(���ؼ�����г�����ʽ����)��
���𰸡�4s24p4 H2S������ O=C=O ![]() ��
��![]() sp2 4
sp2 4 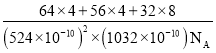 ��
��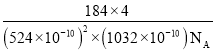
��������
(1)����ԭ�ӵĹ���ԭ����д��̬��ԭ�ӵļ۵����Ų�ʽ������ͬ��Ԫ���γɵĻ��������Է�������Խ�����ʵ��۷е�Խ�ߣ�H2O����֮�������������ʵ��۷е���߷����жϣ�
(2)�ٻ���������ж����м��Լ������ݷ��ӵĿռ乹���ж��Ƿ����ڷǼ��Է��ӣ�����д��ṹ��ʽ�����ȼ���CO2�����ʵ�����Ȼ�����CO2�����к���2��������������������
(3)���ݻ�������ԭ�Ӹ����������γɻ�ѧ���ĵ�����Ŀ��д�������ı�ʾ��
(4)�����ķ���ϵCuFeS2�����ṹ��ʾ������֪��ͭ�����γ��ĸ����ۼ�����ԭ�����������������Ӻ�������ͭ���ӣ��þ�̯��������Ͼ����ṹ����һ�������ں��еĸ���Ԫ�ص�ԭ�Ӹ�����ȷ�������ڹ�CuFeS2����Ŀ��a=b=0.524nm��c=1.032nm��������ܶ�=![]() ���㡣
���㡣
(1)Se��34��Ԫ�أ�����ԭ�Ӻ�������Ų��Ĺ���ԭ������֪���������Ų�ʽ��1s22s22p63s23p63d104s24p4����̬��ԭ�ӵļ۵����Ų�ʽΪ4s24p4������������Ԫ���ǵ�VIA�����⻯�ﻯѧʽͨʽ��H2X����Щ�⻯�ﶼ���ɷ��ӹ��ɣ�����֮��ͨ�����Ӽ���������ϣ����Ӽ�����������Է�������������������Ӽ�������Խ�˷����Ӽ�������ʹ�����������ĵ�������Խ�ߣ����ʵ��۷е��Խ�ߣ�����H2O����֮���������������˷���֮�����������ʹ���۷е���ͬ��Ԫ������ߣ��ʵ�VIA�ļ��⻯���зе���͵���H2S��
(2)���ڷ���ʽ�е����ֻ���������ж����ڼ��Թ��ۼ�������CO2���ɼ��Լ����ɵķǼ��Է��ӣ���ռ乹��Ϊֱ���ͣ��ṹʽ��O=C=O��
��VL��״����CO2�����ʵ�����n(CO2)=![]() mol��������1��CO2�����к���2������������
mol��������1��CO2�����к���2������������![]() molCO2�����к��е�������ĿΪ
molCO2�����к��е�������ĿΪ![]() mol��2��NA/mol=
mol��2��NA/mol=![]() ��
��
(3)��֪ij������Ľṹ��ʽΪ![]() ������ʹ������Ȼ�̼��Һ��ɫ���ɴ���֪���÷����д��ڴ����������ݽṹ��ʽ��֪���γɴ�������ԭ�Ӹ�����5������6�����Ӳ���ɼ�����˿ɱ�ʾΪ��
������ʹ������Ȼ�̼��Һ��ɫ���ɴ���֪���÷����д��ڴ����������ݽṹ��ʽ��֪���γɴ�������ԭ�Ӹ�����5������6�����Ӳ���ɼ�����˿ɱ�ʾΪ��![]() ������Se���ӻ���ʽΪsp2��
������Se���ӻ���ʽΪsp2��
(4)���ݾ����ṹ������֪����������Cu��2��S������������ÿ��Cuԭ����4��S������Cu+����λ��Ϊ4��
�ھ�����Fe2+��Ŀ=8��![]() +4��
+4��![]() +1=4��Cu+����Ŀ=6��
+1=4��Cu+����Ŀ=6��![]() +4��
+4��![]() =4��S2-��ĿΪ8��1=8�����Ծ����ڹ���4��CuFeS2��a=b=524pm��c=1032pm��������ܶ�
=4��S2-��ĿΪ8��1=8�����Ծ����ڹ���4��CuFeS2��a=b=524pm��c=1032pm��������ܶ�![]() =
=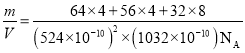 g/cm3��
g/cm3��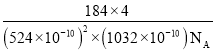 g/cm3��
g/cm3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������������ȷ����(����)
A.����NA����ԭ�ӵĺ����ڱ�״���µ����ԼΪ11.2 L
B.���ʵ���Ũ��Ϊ0.5 mol/L��MgCl2��Һ�к���NA��Cl-
C.��״���£�11.2 L H2O���еķ�����Ϊ0.5 NA
D.�ڳ��³�ѹ�£�1.06 g Na2CO3���е�Na+��Ϊ0.02 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����4 molA�����2mol B������2L�������л�ϣ�����һ�������·������·�Ӧ��2A(g)��B(g)![]() 2C(g)������2s����C��Ũ��Ϊ0.6mol��L��1����
2C(g)������2s����C��Ũ��Ϊ0.6mol��L��1����
��1��������A��ʾ�ķ�Ӧ����Ϊ____��
��2��������B��ʾ�ķ�Ӧ����Ϊ____��
��3��2sʱ����B��ת����Ϊ_____��
��4��2sʱ����A��Ũ��Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NaClO2/H2O2���Ը������ռ���ͬʱ��Ч����������ʵ�����Ʊ�����NaClO2��װ����ͼ��ʾ��װ��I�����¶���35��55��C��ͨ��SO2��NaClO3��ԭΪClO2(�е㣺11��C)��

�ش��������⣺
��1��װ�â��з�Ӧ�����ӷ���ʽΪ__________________��
��2��װ�â��з�Ӧ�Ļ�ѧ����ʽΪ_____________________��
��3��װ������NaOH��Һ��������_____________��
��4�����Ƶõ�NaClO4/H2O2���Ը������ռ�ͬʱ��NO��SO2���������õ�������������ȥ�����¶�һ��ʱ��n(H2O2)/n(NaClO2)����ҺpH������������Ӱ����ͼ��ʾ��

�ٴ�ͼ1��ͼ2�п�֪�������������������n(H2O2)/n(NaClO2)��________________��pH��_________________֮�䡣
��ͼ2��SO2��ȥ������pH�����������NO��ȥ������pH��5.5ʱ������С��NOȥ���ʼ�С�Ŀ���ԭ����___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.��1���������ճ������г�����һЩ���ʣ���Һ�ȡ��ھƾ�����CO2������ʯ�ҡ���̼���ơ���ϡ���ᡢ��CaO����Na2O2����Cu������ָ��(��д���)��
���ڼ������������___���ܵ������___��
��2����������ֱ����___nm֮��
��.��ѧ�����ڻ�ѧ��ռ����Ҫ��λ�����ݼ�����գ�
��1��ʵ����ijŨ�������������Ϊ36.5%���ܶ�Ϊ1.20g��cm��3����Ũ��������ʵ���Ũ����___mol��L��1��
��2��6.8gH2O2���״����___LCO2������ԭ������ͬ��
��.��ȥMg���е�Al�ۣ�ָ��Ӧ������Լ���д���йص����ӷ�Ӧ����ʽ��
Mg��(Al��)���Լ�___�����ӷ���ʽ____��
IV.��ƽ���·���ʽ
___NaClO��___NH3��H2O��___N2H4��___NaCl��___H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��P��QΪ���ֶ�����Ԫ��,��ԭ�Ӱ뾶������������֮��Ĺ�ϵ����ͼ��ʾ������˵����ȷ����

A. Q��������һ���������Ӽ����ۼ� B. ��ۺ����������:Z<Y
C. P����ͼ��⻯�ﳣ�³�ѹ��ΪҺ�� D. Y�γɵĻ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣮

��1����Ũ������HCl�����ʵ���Ũ��Ϊ ______ molL-1��
��2��ȡ����������ĸ�����ʱ�������������в�����ȡ����Ķ��ٶ��仯����______ ��
A����Һ��HCl�����ʵ���
B����Һ��Ũ��
C����Һ��Cl-����Ŀ
D����Һ���ܶ�
��3������ʱ������ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�����ڲ�������հ״������ʵ��������ƣ� ______ ��
A����30mLˮϴ�� ______ 2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע������ƿ��
D��������ƿ�ǽ�����ҡ��
E������ ______ ��ˮ��ʹ��Һ��Һ��ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2cm��
��4�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿������ ��������ƫ��������ƫС����������Ӱ������
a������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�� ______
b�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ ______ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ƾ���(Na2MoO42H2O)��һ����������ȴˮϵͳ�Ľ�����ʴ������ҵ�������⾫��(��Ҫ�ɷ��Dz�����ˮ��MoS2)�Ʊ������Ƶ�����;����ͼ��ʾ��
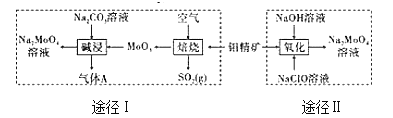
��1��;����ԭ�������������ʵ���֮��Ϊ(���������ʵķ�Ӧ)_____�����ʱ����A�ĵ���ʽΪ_______��
��2��;��II����ʱ��Һ�л���Na2SO4���ɣ���Ӧ�����ӷ���ʽΪ____��
��3����֪;��I����������Һ��c(MoO42-)=0.40 mol/L��c(CO32-)=0.40mol/L������������Һ�Ʊ������ƾ���ʱ�������Ba(OH)2�����Գ�ȥCO32-����CO32-�ij�ȥ��Ϊ90%ʱ����ʽ�����ʱ�Ƿ���BaMoO4��������____��[��֪Ksp(BaCO3)=1��10-9��Ksp(BaMoO4)=4.0��10-8��������Һ������仯]
��4��;��II���õ�BaMoO4��Һ�ᾧ���پ����ؽᾧ��ʹ�����ƾ���Ĵ�����ߣ������õ�ԭ����_____��
��5��ij﮵�طŵ�����У��缫�Ϸ���Lix(MoS2)n��MoS2֮���ת�������طŵ�ʱ�����ĵ缫��ӦʽΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Co(CH3COO)2��4H2O(������)��һ����Ҫ���л�����ԭ�ϡ��ش���������:
(1)�Թ�ҵƷ������(CoO)Ϊԭ���Ʊ������ܡ�(��֪CoO��CH3COOH��Һ��Ӧ����,Co2+����H+��NO3-��������)�����õ����Լ�:Na2CO3��Һ��CH3COOH��Һ��HNO3��Һ���Ƚ�CoO����____(�ѧʽ,��ͬ)��Һ�Ƶ�____��Һ;�ڲ��Ͻ�����,���Ƶõ���Һ�в��ϼ���____��Һ�����ٲ�������,����,����,ϴ��;������м���___��Һ��������ȫ�ܽ�,����pHԼΪ6.8,��һϵ�в�����Co(CH3COO)2��4H2O��
(2)Ϊ̽�������ܵ��ȷֽ����,���ڵ���100 ��ʱʹ����ȥ�ᾧˮ,Ȼ��������װ�ý���ʵ��(��֪CO����PdCl2��Һ��Ӧ���ɺ�ɫPd����):
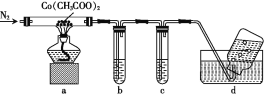
��ͨN2��Ŀ����______��
�ڳ���ʯ��ˮ��PdCl2��Һ�ֱ����ڼ���CO2��CO,����ʢ��PdCl2��Һ��װ����____(����ĸ)��
��ʵ�����ʱ,Ϊ��ֹ����,��ȷ�IJ�����______��
��װ��a����ȫ��Ӧ��õ��ܵ�һ��������,���������(![]() ��100%)Ϊ45.4%����������Ϊ____��
��100%)Ϊ45.4%����������Ϊ____��
��װ��a�ڼ��ȹ�����û��ˮ����,�������ɵĹ�������������Ϊ3.0125 g,װ��b��c�е��Լ�������(b��c�еõ�����������ֱ�Ϊ2.5 g��10.6 g),����ƿ���ռ���������ΪC2H6��N2,��װ��a�з�����Ӧ�Ļ�ѧ����ʽΪ________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com