��������1��ԭ��ط�Ӧ�������Է����еķ��ȵ�������ԭ��Ӧ���ݴ��жϣ�
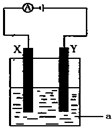
��2������aΪCuSO
4��Һ����X�缫��ͭ���ӷŵ磬��ͭ���ӷŵ���ȫ���������ٷŵ磻Y�缫�����������ӷŵ磻ͨ��һ��ʱ�����������Һ�м���0.2molCu��OH��
2��ĩ��ʹ��Һ�ָ�ԭ״���൱�ڼ���0.2molCuO��0.2molH
2O������������ת�Ƶ���֮��Ĺ�ϵʽ���㣻
������⺬��0.04molCuSO
4��0.04molNaCl�Ļ����Һ400ml���������������ӷŵ�����������ӷŵ磬������������������ȫ�ŵ磬������n��Cl
2��=
n��NaCl��=0.02mol������������������672mL����״���£�ʱ��������������ʵ���=
=0.03mol��0.02mol���������ϻ����������ɣ��������������ʵ���Ϊ0.01mol��������ת�Ƶ��ӵ����ʵ���=2n��Cl
2��+4n��O
2��=0.04mol+0.04mol=0.08mol��������ͭ������ȫ�ŵ�ʱת�Ƶ��ӵ����ʵ���=2��0.04mol=0.08mol�����������������Ӳ��ŵ磬��������������������c��H
+����
���
�⣺��1��ԭ��ط�Ӧ�������Է����еķ��ȵ�������ԭ��Ӧ��
A��C��s��+H
2O��g��=CO��g��+H
2��g����H��0Ϊ���ȷ�Ӧ�����Բ����ϣ��ʴ���
B��NaOH��aq��+HC1��aq��=NaC1��aq��+H
2O��1����H��0Ϊ��������ԭ��Ӧ�����Բ����ϣ��ʴ���
C��2H
2��g��+O
2��g��=2H
2O��1����H��0Ϊ�Է����еķ��ȵ�������ԭ��Ӧ�����Է��ϣ�����ȷ��
��ѡC��
��2������aΪCuSO
4��Һ����X�缫��ͭ���ӷŵ磬��ͭ���ӷŵ���ȫ���������ٷŵ磬Y�缫�����������ӷŵ磬���Ե�ط�Ӧʽ��2CuSO
4+2H
2O
2Cu+O
2��+2H
2SO
4��2H
2O
O
2��+2H
2����ͨ��һ��ʱ�����������Һ�м���0.2molCu��OH��
2��ĩ��ʹ��Һ�ָ�ԭ״���൱�ڼ���0.2molCuO��0.2molH
2O����������������������Cu������ԭ���غ��n��H
2��=n��H
2O��=0.2mol��n��CuO��=n��Cu��=0.2mol��
������0.2molCuת�Ƶ��ӵ����ʵ���Ϊx������0.2mol����ת�Ƶ��ӵ����ʵ���Ϊy��
2CuSO
4+2H
2O
2Cu+O
2��+2H
2SO
4ת�Ƶ���
2mol 4mol
0.2mol x
2mol��4mol=0.2mol��x
x=
=0.4mol
2H
2O
O
2��+2H
2�� ת�Ƶ���
2mol 4mol
0.2mol y
2mol��4mol=0.2mol��y
y=
=0.4mol��
����ת�Ƶ��ӵ����ʵ���=xmol+ymol=0.4mol+0.4mol=0.8mol��
�ʴ�Ϊ��2CuSO
4+2H
2O
2Cu+O
2��+2H
2SO
4��2H
2O
O
2��+2H
2����0.8mol��
������⺬��0.04molCuSO
4��0.04molNaCl�Ļ����Һ400ml���������������ӷŵ�����������ӷŵ磬������������������ȫ�ŵ磬
������n��Cl
2��=
n��NaCl��=0.02mol������������������672mL����״���£�ʱ��������������ʵ���=
=0.03mol��0.02mol��
�������ϻ����������ɣ��������������ʵ���Ϊ0.01mol��������ת�Ƶ��ӵ����ʵ���=2n��Cl
2��+4n��O
2��=0.04mol+0.04mol=0.08mol��
������ͭ������ȫ�ŵ�ʱת�Ƶ��ӵ����ʵ���=2��0.04mol=0.08mol��
���������������Ӳ��ŵ磬����������������ʱ��ͬʱ�������������������ɣ���ط�ӦʽΪ2Cu
2++2H
2O
2Cu+O
2��+4H
+��
���������������ӵĹ�ϵʽ��n��H
+��=4n��O
2��=0.04mol��
��C��H
+��=
=0.1mol/L��
�ʴ�Ϊ��0.1mol/L��

 ��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���
��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

 B��
B��
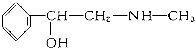 D��
D�� E��
E��

 ��������ֲ��ӷ����е�һ�ֳɷ֣���ṹ��ͼ�������ں����ӵ�����˵����
��������ֲ��ӷ����е�һ�ֳɷ֣���ṹ��ͼ�������ں����ӵ�����˵����