
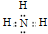 ��N��H��ɵ����ӻ�����һ������笠����ӣ�����Ϊ��NH4H �� NH4��3N��NH4N3��
��N��H��ɵ����ӻ�����һ������笠����ӣ�����Ϊ��NH4H �� NH4��3N��NH4N3��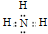 ��NH4H���� NH4��3N��NH4N3����
��NH4H���� NH4��3N��NH4N3����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ϩ�Ľṹ��ʽ��CH2CH2 |
B���ǻ��ĵ���ʽ��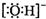 |
C��������ӵı���ģ�ͣ� |
D���Ҵ��Ľṹʽ��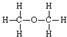 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ �� | �����Ϣ |
| X | ��̬ԭ�Ӻ���ֻ��һ���˶�״̬�ĵ��� |
| Y | ��������ˮ�����������ʻ���ɫ���� |
| Z | ����������Ϊ23��������Ϊ12����������ɫΪ��ɫ |
| W | �����Ĺ�ҵ�������Ľ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ѻ�ģ�� | �ռ������� | ��λ�� |
| bcp | 68% | 8 |
| hcp | 74% | 12 |
| ccp��fcc�� | 74% | 12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
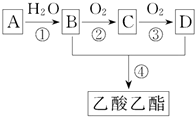 һ�ɵ��������ܸ����Ŀ������ĸо�����Ҫ���㾫���ƾ���ˮ���ɵ���ˮ���ܰ�����ʿ���������㾫���溬���������ʣ���ҵ����AΪ��Ҫԭ�����ϳ�������������ϳ�·����ͼ��ʾ������A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����֪
һ�ɵ��������ܸ����Ŀ������ĸо�����Ҫ���㾫���ƾ���ˮ���ɵ���ˮ���ܰ�����ʿ���������㾫���溬���������ʣ���ҵ����AΪ��Ҫԭ�����ϳ�������������ϳ�·����ͼ��ʾ������A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����֪| ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
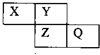 X��Y��Z��Q��Ϊ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Xԭ�ӵ������������ڲ��������2��������˵���У���ȷ���ǣ�������
X��Y��Z��Q��Ϊ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Xԭ�ӵ������������ڲ��������2��������˵���У���ȷ���ǣ�������| A��X��Q�Ļ������в����й��ۼ� |
| B������������Ӧˮ��������ԣ�Q��Zǿ |
| C���⻯���ȶ��ԣ�Y��Zǿ |
| D��Q��Fe��Ӧ���ɵĻ������У���Ԫ����+3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڢۢܢݢ� | B���٢ڢۢܢ� |
| C���ڢۢܢ� | D��ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��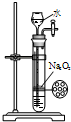 �Ʊ��������� |
B��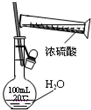 ����һ��Ũ��������Һ |
C��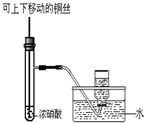 �Ʊ����ռ�����NO2���� |
D��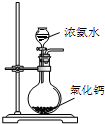 �Ʊ��������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com