
����Ŀ�������仯��������������������Ҫ�����á���ش��������⣺
(1)�£�N2H4��ͨ����������ĸ���ȼ�ϣ���ҵ�ϳ��ô������ƣ�NaClO��������İ�����Ӧ�Ʊ��£��÷�Ӧ�Ļ�ѧ����ʽΪ___��
(2)N2(g)��3H2(g)![]() 2NH3(g)Ϊ���ȷ�Ӧ��800Kʱ��������ʼ�����ͬ���ܱ������г���2molN2��3molH2���������ڷ�Ӧ�����б���ѹǿ���䣬����������������䣬������Ϊ���Ⱥ������������������Խ�����ѧƽ�⡣
2NH3(g)Ϊ���ȷ�Ӧ��800Kʱ��������ʼ�����ͬ���ܱ������г���2molN2��3molH2���������ڷ�Ӧ�����б���ѹǿ���䣬����������������䣬������Ϊ���Ⱥ������������������Խ�����ѧƽ�⡣
�ٴﵽƽ��״̬ʱ��ƽ�ⳣ��K��___K������������������������=������
�ڴﵽƽ��״̬ʱ��N2��Ũ��c��(N2)___c��(N2)��
(3)100kPaʱ����Ӧ2NO(g)+O2(g)![]() 2NO2(g)�е�NO��ƽ��ת�������¶ȵĹ�ϵ������ͼ1��ʾ����Ӧ2NO2(g)
2NO2(g)�е�NO��ƽ��ת�������¶ȵĹ�ϵ������ͼ1��ʾ����Ӧ2NO2(g)![]() N2O4(g)��NO2��ƽ��ת�������¶ȵĹ�ϵ������ͼ2��ʾ��
N2O4(g)��NO2��ƽ��ת�������¶ȵĹ�ϵ������ͼ2��ʾ��
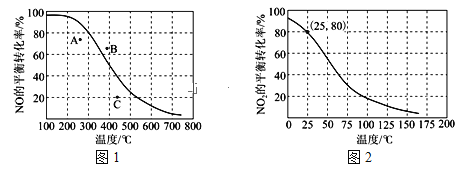
��ͼ1��A��B��C�����ʾ��ͬ�¶ȡ���ͬѹǿ��2NO(g)+O2(g)![]() 2NO2(g)�ﵽƽ��ʱNO��ת���ʣ���___���Ӧ��ѹǿ���
2NO2(g)�ﵽƽ��ʱNO��ת���ʣ���___���Ӧ��ѹǿ���
�ڶ��ڷ�Ӧ2NO2(g)![]() N2O4(g)��д����ƽ���ѹ����ƽ��Ũ�ȼ���ƽ�ⳣ���ı���ʽKp=___������ͼ2������100kPa��25��ʱ��Kp=___��д����ֵ�͵�λ����
N2O4(g)��д����ƽ���ѹ����ƽ��Ũ�ȼ���ƽ�ⳣ���ı���ʽKp=___������ͼ2������100kPa��25��ʱ��Kp=___��д����ֵ�͵�λ����
����ѹ���㣺ij����X�ķ�ѹ��P(X)=��ѹ��X�����ʵ���������
���𰸡�NaClO+2NH3=N2H4+NaCl+H2O = < B ![]() 0.06(kPa)-1
0.06(kPa)-1
��������
(1)�������ƣ�NaClO��������İ�����Ӧ������(N2H4)��NaCl���ɴ˿�д���÷�Ӧ�Ļ�ѧ����ʽ��
(2)�ټס�����������Ϊ�����������¶ȶ�Ϊ800K���¶���ͬʱ��ƽ�ⳣ����ͬ����ɵó�ƽ�ⳣ��K����K���Ĵ�С��ϵ��
����Ϊ���Ǿ��Ⱥ������������ŷ�Ӧ�Ľ��У���������¶����ߡ�������ȣ���������¶ȱ�>�ң����൱���������¶ȣ�ƽ�������ƶ����ɴ˿�ȷ����Ӧ�ﵽƽ��״̬ʱ��N2��Ũ��c��(N2)��c��(N2)�Ĺ�ϵ��
(3)��ͼ1������Ϊ��ͬѹǿ(��Ϊp)����ͬ�¶�ʱNO��ƽ��ת�������¶ȱ仯�����ߣ�A��C��ͬ���µ������ϵĵ���ȣ�NO��ת����С��ӦΪѹǿС��ƽ��ʱ��ѹǿp��B����ͬ�¶��µ������ϵĵ���ȣ�NO��ת���ʴ�ӦΪѹǿ����ƽ��ʱ��ѹǿp���ɴ˿ɵó���Ӧ��ѹǿ���ĵ㡣
�ڶ��ڷ�Ӧ2NO2(g)![]() N2O4(g)��д����ƽ���ѹ����ƽ��Ũ�ȼ���ƽ�ⳣ���ı���ʽKp=
N2O4(g)��д����ƽ���ѹ����ƽ��Ũ�ȼ���ƽ�ⳣ���ı���ʽKp=![]() ������ͼ2���ɼ���n(NO2)=amol����������ʽΪ��
������ͼ2���ɼ���n(NO2)=amol����������ʽΪ��

�ɴ˿ɼ���100kPa��25��ʱ��Kp��
(1)�������ƣ�NaClO��������İ�����Ӧ������(N2H4)��NaCl���ɴ˿�д���÷�Ӧ�Ļ�ѧ����ʽΪNaClO+2NH3=N2H4+NaCl+H2O��
(2)�ټס�����������Ϊ�����������¶ȶ�Ϊ800K���¶���ͬʱ��ƽ�ⳣ����ͬ����ɵó�ƽ�ⳣ��K��=K����
����Ϊ���Ǿ��Ⱥ������������ŷ�Ӧ�Ľ��У���������¶����ߡ�������ȣ���������¶ȱ�>�ң����൱���������¶ȣ�ƽ�������ƶ����ɴ˿�ȷ����Ӧ�ﵽƽ��״̬ʱ��N2��Ũ��c��(N2)<c��(N2)��
(3)��ͼ1�е�����Ϊ��ͬѹǿ(��Ϊp)����ͬ�¶�ʱNO��ƽ��ת�������¶ȱ仯�����ߣ�A��C��ͬ���µ������ϵĵ���ȣ�NO��ת����С��ӦΪѹǿС��������ƽ��ʱ��ѹǿp��B����ͬ�¶��µ������ϵĵ���ȣ�NO��ת���ʴ�ӦΪѹǿ����ƽ��ʱ��ѹǿp���ɴ˿ɵó�ѹǿ���ĵ�ΪB�㣻
�ڶ��ڷ�Ӧ2NO2(g)![]() N2O4(g)����ƽ���ѹ����ƽ��Ũ�ȼ���ƽ�ⳣ���ı���ʽKp=
N2O4(g)����ƽ���ѹ����ƽ��Ũ�ȼ���ƽ�ⳣ���ı���ʽKp=![]() ������ͼ2���ɼ���n(NO2)=amol����������ʽΪ��
������ͼ2���ɼ���n(NO2)=amol����������ʽΪ��

�ɴ˿ɼ���100kPa��25��ʱ��Kp= = 0.06(kPa)-1��
= 0.06(kPa)-1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D����Ԫ�أ����ǵ�ԭ����������������С��18��A��B��ͬһ���ڣ�A�ĵ���ʽΪ��![]() ����Bԭ��L��ĵ���������K���3����0.1 mol C�����ܴ������û���2.24 L(��״��)������ͬʱ���ĵ��Ӳ�ṹ�������ԭ����ͬ�ĵ��Ӳ�ṹ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��
����Bԭ��L��ĵ���������K���3����0.1 mol C�����ܴ������û���2.24 L(��״��)������ͬʱ���ĵ��Ӳ�ṹ�������ԭ����ͬ�ĵ��Ӳ�ṹ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��
(1)д��A��B��C��DԪ�ص����ƣ�A________��B______��C________��D________��
(2)DԪ�������ڱ������ڵ�________����______�塣
(3)�õ���ʽ��ʾA����̬�⻯����γɹ��̣�____________��
(4)A��B�ĵ��ʳ�ַ�Ӧ���ɵĻ�����Ľṹʽ��___________��
(5)B��C�γɵĻ����������ӻ����ﻹ�ǹ��ۻ�������֤����_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ա�Ϊ��Ҫԭ�ϣ���ȡ�߷��Ӳ���N��R���������£�
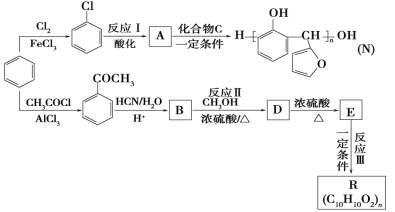
��֪��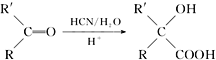
(1)B�к��������ŵ�������___��
(2)��![]() ����
����![]() �ķ�Ӧ������___��
�ķ�Ӧ������___��
(3)һ�������£�������Ӧ�������������Ӧ�Լ���_____��
(4)������C�Ľṹ��ʽ��_____��
(5)��Ӧ��Ļ�ѧ��Ӧ����ʽ��_____��
(6)����˵����ȷ����____ (����ĸ)��
a��A����NaOH��Һ��Ӧ
b�������£�A�ܺ�ˮ������Ȼ���
c��������C��ʹ���Ը��������Һ��ɫ
d��E����˳���칹��
(7)��Ӧ��Ļ�ѧ��Ӧ����ʽ��_____��
(8)��������������B��ͬ���칹����______�֡�
a�����뱥����ˮ��Ӧ���ɰ�ɫ���� b���������� c��������ֻ��������λȡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ����ɵĵ�������ͬ�������ܶ���С�����ʣ���BԪ��ԭ�ӵ����������������ڲ����������2������Dԭ�ӵĵ��Ӳ���������������֮��Ϊ3��1����EԪ�ص������������ǵ��Ӳ�����2������C��Eͬ���塣��ش��������⣺
(1)BԪ�������ڱ��е�λ���ǣ�______________
(2)д��������D2C2�ĵ���ʽ____________���û�������������ѧ������Ϊ____________
(3)������A2C��A2E�У��е�ϸߵ���______________(�ѧʽ)
(4)������EC2�����³���̬������ͨ��Ba(NO3)2��Һ�У��а�ɫ������NO����ų����÷�Ӧ�����ӷ���ʽΪ_______________
(5)Ԫ��A��B��C��ԭ�Ӹ�����2��1��1��ɵĻ������dz���������װ����Ⱦ������ʵķ��ӿռ乹��Ϊ______________���û�������Bԭ�ӵ��ӻ��������Ϊ______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��ɫ��Һ�����п��ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4����K+��CO32����SO42�������ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й�������ͼ��ʾ��

���������ͼ�ƶ���
��1��ԭ��Һ��һ�����ڵ���������_______________����___(����������������������)�ԡ�
��2��ʵ����в�����ɫ��ζ�����������Ļ�ѧ����ʽΪ__________________________________��
��3��д��ʵ�����A���Ӧ�����Ļ�ѧʽ��__________��
��4��д��ʵ���������A��B��������������Ӧ�����ӷ���ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ����ҵ�ϼ��SO2�����Ƿ�ﵽ�ŷű��Ļ�ѧ��Ӧԭ����SO2+ H2O2+ BaCl2= BaSO4��+ 2HC1 ����NA��ʾ�����ӵ�������ֵ������˵������ȷ����
A. 0.1 molBaCl2��������������ԼΪ0.3 NA
B. 25��ʱ��pH=l��HC1��Һ�к���H+����ĿԼΪ0.1 NA
C. ��״���£�17gH2O2��������������ԼΪ9 NA
D. ����2.33gBaSO4����ʱ������SO2������ڱ�״����ԼΪ0.224L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±����и��������У�����ͨ��һ����Ӧʵ����ͼ��ʾת������( )
ѡ�� | X | Y | Z |
A | AlCl3 | Al(OH)3 | NaAlO2 |
B | C | CO | CO2 |
C | CH2=CH2 | CH3CH2Br | CH3CH2OH |
D | S | SO2 | SO3 |
A.AB.BC..CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѪҺ��Ca2����Ũ��һ�����mg��cm��3����ʾ����ȡһ�������Ѫ�����������IJ����[(NH4)2C2O4]��Һ�������������(CaC2O4)���������˲���Ƴ���ϴ�Ӻ�����ǿ��ɵò���(H2C2O4)����������KMnO4��Һ�ζ����ɲⶨѪҺ��Ʒ��Ca2����Ũ�ȡ�ij�о���ѧϰС���������ʵ�鲽��ⶨѪҺ��Ʒ��Ca2����Ũ�ȡ�[��������KMnO4����Һ]��ͼ������50mL����KMnO4����Һ�Ĺ���ʾ��ͼ��
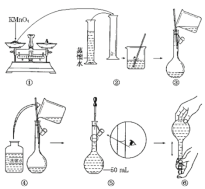
��1������۲�ͼʾ�ж����в���ȷ�IJ�����________(�����)��
��2������ȷ��50mL��Һ�����������__________________________________(������)��
��3���������ͼʾ�IJ��������Ƶ���Һ����ʵ�飬��������������ȷ������£�����õ�ʵ������______(����ƫ��������ƫС��)��
[�ⶨѪҺ��Ʒ��Ca2����Ũ��]��ȡѪ��20.00mL����������������õ����ᣬ����0.020mol��L��1����KMnO4��Һ�ζ���ʹ����ת����CO2�ݳ�����ʱ������12.00mL����KMnO4��Һ��
��4����֪����������KMnO4��Һ��Ӧ�����ӷ���ʽΪ��2MnO4-��5H2C2O4��6H��===2Mn2����10CO2����8H2O�ζ�ʱ����������_______________________________________������ȷ����Ӧ�ﵽ�յ㡣
��5���������㣬ѪҺ��Ʒ��Ca2����Ũ��Ϊ________mg��cm��3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ӧʽ��mX(g)+nY(?)![]() pQ(s)+2mZ(g)����֪��Ӧ�Ѵ�ƽ�⣬��ʱc(X)=0.3mol/L�������������䣬��������С��ԭ����
pQ(s)+2mZ(g)����֪��Ӧ�Ѵ�ƽ�⣬��ʱc(X)=0.3mol/L�������������䣬��������С��ԭ����![]() ��c(X)=0.5mol/L������˵����ȷ���ǣ� ��
��c(X)=0.5mol/L������˵����ȷ���ǣ� ��
A.��Ӧ���淽���ƶ�B.Y�����ǹ����Һ��
C.ϵ��n>mD.Z�����������С
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com