
����Ŀ��I.��CO2ת���ɼ״�ȼ���Ǽ��š�������һ�ֿ�ѧ������
��֪��2H2(g)+O2(g) =2H2O(g)����H=��483.6kJ��mol-1 ��
2CO2(g)+4H2O(g) ![]() 2CH3OH(g)��3O2(g)����H����1352.8kJ��mol-1��
2CH3OH(g)��3O2(g)����H����1352.8kJ��mol-1��
����CO2��H2��Ӧ�Ʊ� CH3OH(g)��ͬʱ����ˮ�������Ȼ�ѧ����ʽΪ___________________
II.���ݻ�Ϊ2L���ܱ������У��������·�Ӧ��A(g)+2B(g)![]() C(g)+D(g)���������1.0molA��2.2molB���ڲ�ͬ�¶��£�D�����ʵ���n(D)��ʱ��t�Ĺ�ϵ��ͼ��
C(g)+D(g)���������1.0molA��2.2molB���ڲ�ͬ�¶��£�D�����ʵ���n(D)��ʱ��t�Ĺ�ϵ��ͼ��

�Իش��������⣺
(1)800��ʱ,0��5min�ڣ���B��ʾ��ƽ����Ӧ����Ϊ____________��
(2)���жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������_______________��
a��������ѹǿ���� b�����������c(A)����
c��2v��(B)=v��(D) d��c(A)=c(C)
(3)���������1.0molA��2.2molB������ͼ�����ݼ���800��ʱ��ƽ�ⳣ��K=________���÷�ӦΪ_______��Ӧ(�����Ȼ����),�ж�������______________________________��
(4)800��ʱ��ijʱ�̲����ϵ�и����ʵ������£�n(A)=0.9mol��n(B)=2.0mol��n(C)=0.9mol��n(D)=0.9mol�����ʱ�÷�Ӧ________����(����������Ӧ�����������淴Ӧ��������������ƽ��״̬��)��
���𰸡�CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g) ��H��-49 kJ��mol��1 0.12mol��L-1��min-1 ab 1.8 L/mol ���� �����¶ȵ����ߣ�D�ĺ������࣬������������ԭ�����¶����������������ȷ������ ������Ӧ����
CH3OH(g)��H2O(g) ��H��-49 kJ��mol��1 0.12mol��L-1��min-1 ab 1.8 L/mol ���� �����¶ȵ����ߣ�D�ĺ������࣬������������ԭ�����¶����������������ȷ������ ������Ӧ����
��������
I.���ݸ�˹������дCO2��H2��Ӧ�Ʊ� CH3OH(g)���Ȼ�ѧ����ʽ��
II. (1)����![]() ����v(B)��
����v(B)��
(2)����ƽ���־�����÷�Ӧ�Ƿ�ﵽƽ��״̬��
(3)���� ![]() ����ƽ�ⳣ���� ����ͼ�����¶ȣ�D�ĵĺ������٣�˵������ƽ�������ƶ���
����ƽ�ⳣ���� ����ͼ�����¶ȣ�D�ĵĺ������٣�˵������ƽ�������ƶ���
(4)����Q��K�Ĺ�ϵ�ж�n(A)=0.9mol��n(B)=2.0mol��n(C)=0.9mol��n(D)=0.9molʱ�÷�Ӧ�ķ���
I.2H2(g)+O2(g) =2H2O(g)����H=��483.6kJ��mol-1 ��
2CO2(g)+4H2O(g) ![]() 2CH3OH(g)��3O2(g)����H����1352.8kJ��mol-1��
2CH3OH(g)��3O2(g)����H����1352.8kJ��mol-1��
���ݸ�˹���ɢ���2����![]() �� CO2(g)��3H2(g)
�� CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g) ��H��-49 kJ��mol��1��
CH3OH(g)��H2O(g) ��H��-49 kJ��mol��1��
II. (1) 800��ʱ,0��5min�ڣ�����D 0.6mol��������B 1.2mol�� v(B)= ![]() =
=![]() 0.12mol��L��1��min��1��
0.12mol��L��1��min��1��
(2) a��A(g)+2B(g)![]() C(g)+D(g)��Ӧǰ���������ʵ����DZ�����ѹǿ�DZ�����������ѹǿ����һ���ﵽƽ��״̬�� b�����ݻ�ѧƽ�ⶨ�壬ij����Ũ�Ȳ���ﵽƽ��״̬�����������c(A)����һ���ﵽƽ��״̬�� c��2v��(B)=v��(D) �����淴Ӧ���ʱȲ�����ϵ���ȣ���Ӧû��ƽ�� �� d��c(A)=c(C)��Ũ�Ȳ�һ�����䣬��Ӧ��һ��ƽ�⣬��ѡab��
C(g)+D(g)��Ӧǰ���������ʵ����DZ�����ѹǿ�DZ�����������ѹǿ����һ���ﵽƽ��״̬�� b�����ݻ�ѧƽ�ⶨ�壬ij����Ũ�Ȳ���ﵽƽ��״̬�����������c(A)����һ���ﵽƽ��״̬�� c��2v��(B)=v��(D) �����淴Ӧ���ʱȲ�����ϵ���ȣ���Ӧû��ƽ�� �� d��c(A)=c(C)��Ũ�Ȳ�һ�����䣬��Ӧ��һ��ƽ�⣬��ѡab��
(3)
A(g)+2B(g)![]() C(g)+D(g)
C(g)+D(g)
��ʼ 0.5 1.1 0 0
ת�� 0.3 0.6 0.3 0.3
ƽ�� 0.2 0.5 0.3 0.3
K=![]() 1.8 L/mol��
1.8 L/mol��
����ͼ�����¶ȣ� D�ĺ������٣�˵������ƽ�������ƶ�������Ӧ���ȣ�
(4) c(A)=0.45mol/L��c(B)=1.0mol/L��c(C)=0.45mol/L��c(D)=0.45mol/L��Q=![]() 0.45��1.8������������Ӧ������С�
0.45��1.8������������Ӧ������С�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ܡ��ѡ�ͭ��Ԫ�س������Ʊ�����ӵ�ص��������ϻ��Ч������NA��ʾ�����ӵ�����������д���пհס�
��1����̬Coԭ�ӵĵ����Ų�ʽΪ___��
��2������CO���ɵ������Ni(CO)4�У����ṩ�µ��ӶԵijɼ�ԭ����___����Ԫ�����ƣ���1 molNi(CO)4�к��е�������ĿΪ__��д����CO��Ϊ�ȵ������һ�������ӵĻ�ѧʽ_____��
��3��Ti(BH4)2��һ�ִ�����ϡ�BH4-�Ŀռ乹����____��Bԭ�ӵ��ӻ���ʽ__������ͬ���ڵĵڢ�B��͢�A������Ԫ���е�һ�����ܽϴ����___��дԪ�ط��ţ���ԭ����____��
��4��CuFeS2�ľ�����ͼ��ʾ�����������ֱ�Ϊanm��bnm��cnm��CuFeS2�ľ�����ÿ��Cuԭ����___��Sԭ�������������ܶ�����___g��cm3���г��������ʽ����

�Ծ�������Ϊ��λ���Ƚ���������ϵ���Ա�ʾ�����и�ԭ�ӵ�λ�ã�����ԭ�ӷ������꣬����ͼ��ԭ��2��3������ֱ�Ϊ��0��1��![]() ������
������![]() ��
��![]() ��0������ԭ��1������Ϊ___��
��0������ԭ��1������Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʾ��ͼ�У����������ԭ�ӣ����������ԭ�ӣ�������������������м���һ���������»����ĸ��壨���������Բ��ƣ��������ܱ�ʾ�������������뺤�����ǣ�������
A.  B.
B.  C.
C.  D.
D. 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������Ͳ��ȡ50 mL 0.25 mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50 mL 0.55 mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ��۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȡ�
�ش��������⣺
(1)д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ(�к�����ֵΪ57.3 kJ/mol)��_______________________________________________________________
(2)ѧ������ϡ������ϡ�ռ���Һ�ⶨ�к���װ����ͼ��

��ʵ��ʱ����Ҫ�IJ����������ձ�����Ͳ���Ҫ��_________��
�ڸ�װ������һ�������ǣ�______________________��
(3)����ͬŨ�Ⱥ�����İ�ˮ(NH3�� H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��________(����ƫ��������ƫС��������Ӱ����)��
��.(1)��֪���ȼ��һ�������Ķ���(C4H10)����ʱ����1mol������̼�����Һ̬ˮ�����ų�����bkJ�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ______________________
(2)��֪�����Ȼ�ѧ����ʽ��
C(s��ʯī)+O2(g)�TCO2(g)��H=-393.5kJmol-1
2H2(g)+O2(g)�T2H2O(l)��H=-571.6kJmol-1
2C2H2(g)+5O2(g)�T4CO2(g)+2H2O(l)��H=-2599kJmol-1
��д��C(s��ʯī)��H2(g)����1mol C2H2(g)���Ȼ�ѧ����ʽ____________________
(3)��֪���ֹ��ۼ��ļ����������±���
���ۼ� | N��N | H��H | N��H |
���� (kJ/mol) | 946 | 436 | 390.8 |
д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ�� ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ�ת���ڸ�����������ʵ�ֵ���
A. Na![]() Na2O
Na2O![]() Na2CO3
Na2CO3
B. Al![]() Al2O3
Al2O3![]() Al(OH)3
Al(OH)3
C. Fe(OH)2![]() Fe(OH)3
Fe(OH)3![]() Fe2O3
Fe2O3
D. Na2CO3(aq)![]() NaHCO3
NaHCO3![]() CO2
CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС���ͬѧ���ʵ���Ʊ�CuBr(��ɫ�ᾧ�Է�ĩ������ˮ���������Ҵ����л��ܼ�)��ʵ��װ��(�г֡�����������)��ͼ��ʾ��
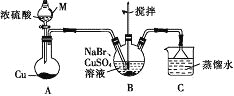
(1)����M��������________��
(2)����M�е�Ũ���ỻ��70%��H2SO4����Բ����ƿ�еĹ����Լ�Ϊ______(�ѧʽ)��
(3)B�з�����Ӧ�Ļ�ѧ����ʽΪ_______����˵��B�з�Ӧ����ɵ�������_____����B��Cu2+��δ��ȫ����ԭ�����˼�����Լ���_______(����)��
a.Һ�� b.Na2SO4 c.���� d.Na2S2O3
(4)���й��ڹ��˵���������ȷ����_______ (����)��
a.©��ĩ�˾�����Բ������ձ���
b.����ֽ��ʪ��ʹ�����©���ڱ�
c.��ֽ��Ե���Ը߳�©����
d.�ò�������©������������Լӿ��������
(5)ϴ��ʱ������װ��C�е�����Һ��ϴ����Ŀ����_______�����������ܽ�SO2���Ҵ�������ϴ�ӵ�Ŀ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ھ����˵��һ����ȷ���ǣ�������
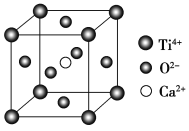
(CaTiO3�ľ���ṹģ��(Ca2����O2����Ti4���ֱ�λ������������ġ����ĺͶ���)
A. ���Ӿ����ж����ڹ��ۼ�
B. CaTiO3������ÿ��Ti4����12��O2�������
C. SiO2������ÿ����ԭ����������ԭ���Թ��ۼ�����
D. ����������۵㶼�ȷ��Ӿ�����۵��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ͼװ����ȡ������������������ʵ�ʵ�顣
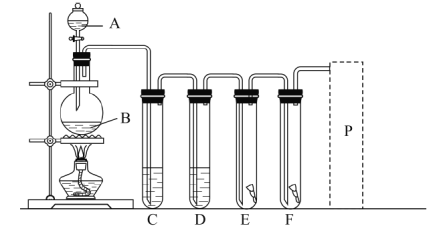
(1)A��B�����������ƣ�A________��B________��
(2)д��ʵ�����ô�װ����ȡ�����Ļ�ѧ����ʽ________��
(3)Ϊ�˵õ����������������д��װ��C��D��Ӧ�ü����ҩƷ�����ƣ�C____��D_________��
(4)E�з����ɫ�ɲ�����F�з����ɫʪ�������ɹ۲쵽��������________��д��������Ӧ�Ļ�ѧ����ʽ________��
(5)P��Ϊβ������װ�ã�����װ��ͼ��������õ�ҩƷ��________

(6)NaClO����84������Һ����Ч�ɷ֡���ش��������⡣
��NaClO��ClԪ�صĻ��ϼ���______��
��������������Һ��������Ӧ�Ʊ���84������Һ����Ӧ�����ӷ���ʽ��_______��
��������84������Һ�����飨��Ҫ�ɷ������ᣩ���ʹ�ã�����������ɫ���ж����壬�䷴Ӧ�����ӷ���ʽ��_________��
(7)FeCl3���ִ���ҵ��Ӧ�ù㷺��ij��ѧ�о���ѧϰС��ģ�ҵ���������Ʊ���ˮFeCl3�����������ϵ�֪����ˮFeCl3�ڿ������׳��⣬��������������ˮCaCl2�dz��õĸ����������������Ʊ���ˮFeCl3��ʵ�鷽����װ��ʾ����ͼ�����ȡ��ӳּ�β������װ����ȥ���������������£�
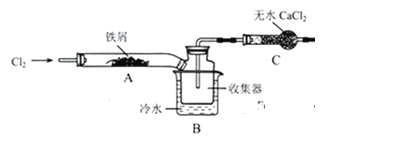
�ټ��װ��������
��ͨ�������������Ͼ�װ���ڵĿ���
���þƾ�������м�·���������Ӧ���
������
����ϵ��ȴ��ֹͣͨ�����������ø���ĵĵ����Ͼ����������ռ����ܷ⡣
��ش��������⣺
��װ��A�з�����Ӧ�Ļ�ѧ����ʽ_____________��
�ڲ���ۼ��Ⱥ����ɵ���״FeCl3�ֽ����ռ��������������ڷ�Ӧ��A���Ҷˡ�Ҫʹ������FeCl3�����ռ������ڢܲ�������________��
�۲��������У�Ϊ��ֹ��������ȡ�Ĵ�ʩ��(������)________��
��װ��B�е���ȴˮ����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ǧ��ҵ���ҹ������ӻ��������ͳ��������ɫ����������Դ���������չ���������˲�ҵ���ӷϾ�Ǧ�����л���Ǧ�Ĺ���Ϊ��
![]()
���ԭ����ͼ��ʾ������˵����ȷ����
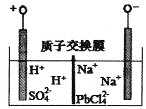
A. �������缫��ӦʽΪ��2H++2e��=H2��
B. ����������������pH��������
C. Na2PbCl4Ũ���½���������������PbO����ʵ�ֵ������Һ������ʹ��
D. ��·������4mol���ӣ������ɵ�207gǦ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com