
����Ŀ��I.������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������Ͳ��ȡ50 mL 0.25 mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50 mL 0.55 mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ��۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȡ�
�ش��������⣺
(1)д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ(�к�����ֵΪ57.3 kJ/mol)��_______________________________________________________________
(2)ѧ������ϡ������ϡ�ռ���Һ�ⶨ�к���װ����ͼ��

��ʵ��ʱ����Ҫ�IJ����������ձ�����Ͳ���Ҫ��_________��
�ڸ�װ������һ�������ǣ�______________________��
(3)����ͬŨ�Ⱥ�����İ�ˮ(NH3�� H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��________(����ƫ��������ƫС��������Ӱ����)��
��.(1)��֪���ȼ��һ�������Ķ���(C4H10)����ʱ����1mol������̼�����Һ̬ˮ�����ų�����bkJ�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ______________________
(2)��֪�����Ȼ�ѧ����ʽ��
C(s��ʯī)+O2(g)�TCO2(g)��H=-393.5kJmol-1
2H2(g)+O2(g)�T2H2O(l)��H=-571.6kJmol-1
2C2H2(g)+5O2(g)�T4CO2(g)+2H2O(l)��H=-2599kJmol-1
��д��C(s��ʯī)��H2(g)����1mol C2H2(g)���Ȼ�ѧ����ʽ____________________
(3)��֪���ֹ��ۼ��ļ����������±���
���ۼ� | N��N | H��H | N��H |
���� (kJ/mol) | 946 | 436 | 390.8 |
д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ�� ____________________________________��
���𰸡�![]() H2SO4(aq)��NaOH(aq)��
H2SO4(aq)��NaOH(aq)��![]() Na2SO4(aq)��H2O(l)��H����57.3 kJ/mol �¶ȼơ����β�������� ���ձ���û�и���Ӳֽ�� ƫС C4H10(g)��13/2O2(g)��4CO2(g)��5H2O(l)����H��-4bkJ/mol 2C(s��ʯī)+H2(g)=C2H2(g) ��H=+226.7 kJmol-1 N2(g)��3H2(g)
Na2SO4(aq)��H2O(l)��H����57.3 kJ/mol �¶ȼơ����β�������� ���ձ���û�и���Ӳֽ�� ƫС C4H10(g)��13/2O2(g)��4CO2(g)��5H2O(l)����H��-4bkJ/mol 2C(s��ʯī)+H2(g)=C2H2(g) ��H=+226.7 kJmol-1 N2(g)��3H2(g)![]() 2NH3(g) ��H����90.8 kJ��mol-1
2NH3(g) ��H����90.8 kJ��mol-1
��������
��1���к�����ǿ���ǿ���ϡ��Һ��ȫ��Ӧ����1molˮ�ų��������������Ȼ�ѧ����ʽ����д����д���Ȼ�ѧ����ʽ��
��2���ٲ����к�����Ҫ������Һ���¶��Լ���Ӧ����Ҫ���裻
�ڸ��ݲ����к�����Ҫ�����ܷ�ֹ����ɢʧ������
(3)��ˮΪ����������Ϊ���ȹ��̣�
��.(1)ȼ������ָ1mol��������ȫȼ�������ȶ���������ų���������
(2)���ݸ�˹������дC(s��ʯī)��H2(g)����1mol C2H2(g)���Ȼ�ѧ����ʽ��
(3)�ʱ�=��Ӧ���ܼ��ܣ��������ܼ��ܣ�
��1��ǿ�ᡢǿ����к���Ϊ-57.3kJ/mol���к�����ǿ���ǿ���ϡ��Һ��ȫ��Ӧ����1molˮ�ų���������ϡ�����ϡ����������Һ��Ӧ���Ȼ�ѧ����ʽΪ��![]() H2SO4(aq)��NaOH(aq)��
H2SO4(aq)��NaOH(aq)��![]() Na2SO4(aq)��H2O(l) ��H����57.3 kJ/mol��
Na2SO4(aq)��H2O(l) ��H����57.3 kJ/mol��
��2����������Ҫ������Һ���¶��Լ���Ӧ����Ҫ���裬���Ի�ȱ�ٵIJ����������¶ȼơ����β����������
��װ��ͼ��û����Ӳֽ�壨�����ϰ壩��ס�ձ��������ͻᵼ����������ʧ�����Ը�װ�õĴ����Ǵ��ձ���û�и���Ӳֽ�塣
(3)��ˮΪ����������Ϊ���ȹ��̣�����ͬŨ�Ⱥ�����İ�ˮ(NH3�� H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��ƫС��
��.(1)���ȼ��һ�������Ķ���(C4H10)����ʱ����1mol������̼�����Һ̬ˮ�����ų�����bkJ��1mol����(C4H10)������ȫȼ�����ɶ�����̼�����Һ̬ˮ�����ų�����4bkJ������ȼ���ȵ��Ȼ�ѧ����ʽΪC4H10(g)��13/2O2(g)��4CO2(g)��5H2O(l)����H��-4bkJ/mol��
(2) C(s��ʯī)+O2(g)�TCO2(g)��H=-393.5kJmol-1
2H2(g)+O2(g)�T2H2O(l)��H=-571.6kJmol-1
2C2H2(g)+5O2(g)�T4CO2(g)+2H2O(l)��H=-2599kJmol-1
���ݸ�˹������2��![]() 2��
2��![]() 2��2C(s��ʯī)+H2(g)=C2H2(g) ��H=+226.7 kJmol-1��
2��2C(s��ʯī)+H2(g)=C2H2(g) ��H=+226.7 kJmol-1��
(3) �ʱ�=��Ӧ���ܼ��ܣ��������ܼ��ܣ��ϳɰ���Ӧ���Ȼ�ѧ����ʽN2(g)��3H2(g) ![]() 2NH3(g) ��H��(946��436��3��390.8��6)kJ��mol-1����90.8 kJ��mol-1��
2NH3(g) ��H��(946��436��3��390.8��6)kJ��mol-1����90.8 kJ��mol-1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ȼ��ѣ�TiCl4������ȡ���ղ��������ѺϽ����Ҫԭ�ϡ�����������Ҫ�ɷ���FeTiO3��������ˮ���Ʊ�TiCl4�Ȳ�Ʒ��һ�ֹ�������ʾ����ͼ��
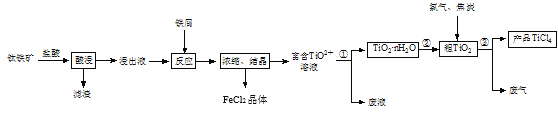
�ش��������⣺
��1���������ʱ��Ҫ����������飬��Ŀ����____��
��2������Һ��ǿ���ԣ�����TiO2����Fe2��������Fe3����Al3�������ӣ�������TiO2�������ӷ���ʽ��__��
��3������Ӧ��ʱ�������м������Һ����ɫ����ʱ��Һ�Գ�ǿ���ԡ��ù����������·�Ӧ������
2Fe3����Fe��3Fe2��
2TiO2������ɫ����Fe��4H����2Ti3������ɫ����Fe2����2H2O
Ti3������ɫ����Fe3����H2O��TiO2������ɫ����Fe2����2H��
�������������____��
��4��ͨ����������������ʹ���̢������ɵ�TiO2��nH2O�γ�һ��Һ̬��ɢϵ����һ��������÷�ɢϵ�ܹ����������ЧӦ�����ɢ�ʿ���ֱ���ķ�Χ��____��
��5�����̢��ƵõĹ���TiO2��nH2O���ù�������ϴ�Գ�ȥ���е�Fe(OH)3���ʣ�����Fe(OH)3���ʳ�����ʵ�鷽����____��
��6�����̢����ɵķ����к���CO����TiO2�ͽ�̿�������ڸ����·�����Ӧ�Ļ�ѧ����ʽ��___��
��7���������վ��гɱ��͡����õ�Ʒλ����Ϊԭ�ϵ��ŵ㡣������ɫ��ѧ����ù��������д��ڵIJ���֮����____��ֻҪ��д��һ���
��8�����ݱ�����Ϣ���ɲ���___�������ƺ�����SiCl4���ʵ�TiCl4��
TiCl4 | SiCl4 | |
�۵�/�� | ��25.0 | ��68.8 |
�е�/�� | 136.4 | 57.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о�����ȿ���CO��CO2��Ӧ�þ�����Ҫ��������壮�ش��������⣺
I��CO�����ڸ�¯��������֪��
Fe3O4(s)+4CO(g)=3Fe(s)+4CO2 (g) ��H1= a kJ/mol
3Fe2O3(s)+CO(g)=2Fe3O4(s)+CO2 (g) �� H2= b kJ/mol
��ӦFe2O3(s)+3CO(g)=2Fe(s)+3CO2 (g) ����H= ______________________________kJ/mol
II��ij�¶��£����ݻ�Ϊ2 L���ܱ���������Ͷ��8molCO2(g)��16molH2(g)������Ӧ��
CO2 (g)+H2(g) ![]() CO(g)+H2O(g) ������15 min��ﵽƽ�⣬��ʱCO2��ת����Ϊ75%����015 min���ö�����̼��ʾƽ����Ӧ����v��CO2��= _________________������������¸÷�Ӧ��ƽ�ⳣ��K= _________________��
CO(g)+H2O(g) ������15 min��ﵽƽ�⣬��ʱCO2��ת����Ϊ75%����015 min���ö�����̼��ʾƽ����Ӧ����v��CO2��= _________________������������¸÷�Ӧ��ƽ�ⳣ��K= _________________��
III����̼������ָ�ӿ����в��������̼�ĸ��ֿ�ѧ������ͳ�ơ�ĿǰNH3��(NH4)2CO3�������Ѿ���������ҵ��̼����
��1�����������в�������ΪCO2���������___________��
A.Na2CO3 B.NaOH C.CH3CH2OH D.NH4Cl
��2����(NH4)2CO3��̼�ķ�Ӧ��(NH4)2CO3(aq)��H2O(l)��CO2(g) ![]() 2NH4HCO3(aq)��Ϊ�о��¶ȶ�(NH4)2CO3����CO2Ч�ʵ�Ӱ�죬��һ������(NH4)2CO3��Һ�����ܱ������У�������һ������CO2���壬����������ʼʵ���������䣬�ֱ��ڲ�ͬ�¶��£�������ͬʱ����CO2����Ũ�ȣ��õ�����ͼ��
2NH4HCO3(aq)��Ϊ�о��¶ȶ�(NH4)2CO3����CO2Ч�ʵ�Ӱ�죬��һ������(NH4)2CO3��Һ�����ܱ������У�������һ������CO2���壬����������ʼʵ���������䣬�ֱ��ڲ�ͬ�¶��£�������ͬʱ����CO2����Ũ�ȣ��õ�����ͼ��
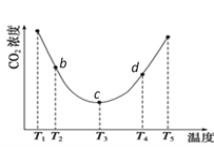
��c����淴Ӧ���ʺ�d�������Ӧ���ʵĴ�С��ϵΪ����c _____����d (������������������������)
��b��c��d�����ƽ�ⳣ��K b ��K c�� Kd �Ӵ�С��˳��Ϊ ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
(1)48g������48g����(O3)������������ԭ����_______ (������ͬ��������ͬ����������ͬ��ͬѹ�µ��������________��0.2 mol NH3������________��CH4���еĵ�������ͬ����_______g H2O���е���ԭ������ͬ�����״����____ L CO���е�ԭ������ȡ�
(2)483g Na2SO4��10H2O��������Na2SO4��10H2O�����ʵ�����_______�� Na2SO4��10H2O��Ħ��������________������Na+�����ʵ�����________����0.4 mol Al3����Al2(SO4)3��������SO42-�����ʵ�����________��
(3)ʵ���ҳ��õ�Ũ�����ܶ�Ϊ1.17 g��mL-1����������Ϊ36.5 %��
�ٴ�Ũ��������ʵ���Ũ��Ϊ__________________��
��ȡ��Ũ����50mL��������ˮϡ��Ϊ200mL��ϡ�ͺ���������ʵ���Ũ��Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ѧ�ҷ��֣�ʳ��Ϻ���ˮ�����ද���ͬʱ����ά����C�����ж���������Ϊ����������+5�����������ά����C���������ܹ�ת��Ϊ�ж���+3�۵ĺ��黯���ͨ��������Ϣ��գ�����������+5������������Ϊ______������������ԭ������+5����Ԫ�ط���______��Ӧ����������ԭ����0.5mol+5������ȫת��Ϊ+3���飬��ת��______�����ӡ�
��2��ʵ���ҳ������·�����ȡ������MnO2+4HCl(Ũ)![]() MnCl2+Cl2��+2H2O��
MnCl2+Cl2��+2H2O��
����˫���ű������ת�Ʒ������Ŀ______�����ڴ���ֽ�ϱ꣩
���ڱ�״�������ɵ�����Ϊ11.2Lʱ����������HCl������Ϊ______��
��Ϊ��֤ʵ�鰲ȫ����������������Һ���ն����������д����Ӧ�����ӷ���ʽ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ����ʽ�У�������ȷ���ﷴӦ��ɫ�仯��ԭ�����
A. ͭ���ÿ����б��������ɫ���壺2Cu��O2��CO2��H2O�TCu2(OH)2CO3
B. ij�ֻ�������������N2O4���ݳ���������ɫ���壺N2O4![]() 2NO2
2NO2
C. FeSO4��7H2O�ڿ����о��ñ�ƣ�2FeSO4��7H2O![]() Fe2O3��SO2����SO3����14H2O
Fe2O3��SO2����SO3����14H2O
D. SO2ͨ��KMnO4��Һ�У���Һ��ɫ����ȥ��5SO2��2KMnO4��2H2O�TK2SO4��2MnSO4ʮ2H2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.��CO2ת���ɼ״�ȼ���Ǽ��š�������һ�ֿ�ѧ������
��֪��2H2(g)+O2(g) =2H2O(g)����H=��483.6kJ��mol-1 ��
2CO2(g)+4H2O(g) ![]() 2CH3OH(g)��3O2(g)����H����1352.8kJ��mol-1��
2CH3OH(g)��3O2(g)����H����1352.8kJ��mol-1��
����CO2��H2��Ӧ�Ʊ� CH3OH(g)��ͬʱ����ˮ�������Ȼ�ѧ����ʽΪ___________________
II.���ݻ�Ϊ2L���ܱ������У��������·�Ӧ��A(g)+2B(g)![]() C(g)+D(g)���������1.0molA��2.2molB���ڲ�ͬ�¶��£�D�����ʵ���n(D)��ʱ��t�Ĺ�ϵ��ͼ��
C(g)+D(g)���������1.0molA��2.2molB���ڲ�ͬ�¶��£�D�����ʵ���n(D)��ʱ��t�Ĺ�ϵ��ͼ��

�Իش��������⣺
(1)800��ʱ,0��5min�ڣ���B��ʾ��ƽ����Ӧ����Ϊ____________��
(2)���жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������_______________��
a��������ѹǿ���� b�����������c(A)����
c��2v��(B)=v��(D) d��c(A)=c(C)
(3)���������1.0molA��2.2molB������ͼ�����ݼ���800��ʱ��ƽ�ⳣ��K=________���÷�ӦΪ_______��Ӧ(�����Ȼ����),�ж�������______________________________��
(4)800��ʱ��ijʱ�̲����ϵ�и����ʵ������£�n(A)=0.9mol��n(B)=2.0mol��n(C)=0.9mol��n(D)=0.9mol�����ʱ�÷�Ӧ________����(����������Ӧ�����������淴Ӧ��������������ƽ��״̬��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Һ���Ǹ������ʣ������ܵ��������塣����˵������ȷ����(����)
A. NH3�����е�ԭ�ӵ��ӻ���ʽΪsp3�ӻ�
B. [Cu(NH3)4]2����NH3����������
C. NH![]() ��PH
��PH![]() ��CH4��BH
��CH4��BH![]() ��Ϊ�ȵ�����
��Ϊ�ȵ�����
D. ��ͬѹǿ�£�NH3�ķе��PH3�ķе��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ԭ��Ӧ��һ����Ҫ�ķ�Ӧ���ڹ�ũҵ�������ճ������ж��й㷺����;��
(1)��ҩ���й��ġ��Ĵ�����֮һ����Զֵ�������セ�����ڻ�ҩ�ڷ�����ըʱ���������·�Ӧ��2KNO3��3C��S=K2S��N2����3CO2�������б�������Ԫ����____________����ԭ������____________��
(2)ʵ����Ϊ�������й������ĺ�������������Ϳ��CuI����ֽ��������ֽ�Ƿ��ɫ����ɫ�����仯����ȥ��ʱ�����жϿ����еĺ��������䷴ӦΪ4CuI��Hg=Cu2HgI4��2Cu��
��������Ӧ����Cu2HgI4�У�CuԪ����________�ۡ�
�����Ϸ�Ӧ�е�������Ϊ________������1 mol CuI���뷴Ӧʱ��ת�Ƶ���________mol��
�۱���������Ӧ����ת�Ƶķ������Ŀ��____________________________��
(3)��ҵ�ϳ������Ը��������Һ��������CuS��Cu2S�Ŀ���䷴Ӧԭ�����£�
8MnO4����5Cu2S��44H��=10Cu2����5SO2����8Mn2����22H2O
6MnO4����5CuS��28H��=5Cu2����5SO2����6Mn2����14H2O
����������Ӧԭ����ijѧϰС����400 mL 0.075 mol��L��1�����Ը��������Һ����2 g����CuS��Cu2S�Ļ�����Ӧ�������Һ���Ͼ�SO2��ʣ���KMnO4ǡ����350 mL 0.1 mol��L��1��(NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
����ƽKMnO4��(NH4)2Fe(SO4)2��Ӧ�����ӷ���ʽ��______��MnO![]() ��Fe2����H��=Mn2����Fe3����H2O
��Fe2����H��=Mn2����Fe3����H2O
��KMnO4��Һ��������ﷴӦ��ʣ��KMnO4�����ʵ���Ϊ________ mol��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com