
| 120�� | 300�� | 480�� | |
| ��ѧʽ | KxFey��C2O4��Z | KxFeyO��C2O4��Z-1 | KxFeyO2��C2O4��Z-2 |
| ���� | 4.370g | 3.650g | 2.930g |
| m |
| n |
| 0.54g |
| 4.91g |
| 0.72g |
| 72g/mol |
| 4.91g |
| 0.01mol |
| 0.8g |
| 160g/mol |
| 491-39��3-88��3 |
| 18 |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��mNA | ||
B��
| ||
C��
| ||
D��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ձ����������������ձ��� |
| B�����ձ��е���һ������ |
| C�����ձ��вμӷ�Ӧ��п���� |
| D����Ӧ��ʼʱ���ձ��в������������ʱȼ��ձ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
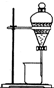 ijͬѧΪ����֤�����к��е�Ԫ�ز���ȡ�ⵥ�ʣ����������ʵ�飬��ش�������⣮
ijͬѧΪ����֤�����к��е�Ԫ�ز���ȡ�ⵥ�ʣ����������ʵ�飬��ش�������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��һ��������þ���������Ͷ�뵽һ�����1mol?L-1�������У���������ȫ�ܽ������Һ�м���1mol?L-1������������Һ�����ɵij������������������Һ�������ϵ��ͼ��ʾ������ù��������ɳ����������Ϊ0.03mol��
��һ��������þ���������Ͷ�뵽һ�����1mol?L-1�������У���������ȫ�ܽ������Һ�м���1mol?L-1������������Һ�����ɵij������������������Һ�������ϵ��ͼ��ʾ������ù��������ɳ����������Ϊ0.03mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������ʻ�ʩ����S-�տ����Ƽ��ɱ�֤�ʻ�ʢ����S-�տ��صķ��ӽṹ��ͼ������˵����ȷ���ǣ�������
�������ʻ�ʩ����S-�տ����Ƽ��ɱ�֤�ʻ�ʢ����S-�տ��صķ��ӽṹ��ͼ������˵����ȷ���ǣ�������| A�����ܷ����Ӿ۷�Ӧ�����ܷ������۷�Ӧ |
| B��1 mol��������NaOH��Һ��Ӧ���������2 mol NaOH |
| C�������ʽΪC15H22O4 |
| D���ȿ�����FeCl3��Һ������ɫ��Ӧ���ֿ���ʹ����KMnO4��Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com