
����Ŀ��A��G����ѧ��ѧ�������ʣ�A��DΪ���ʣ�G�Ǻ�AԪ�ص��������塣��֪��A(s)+B(g)=C(g)+D(g) ��H= +131.4 kJmol-1��ijͬѧʵ���֪��4 g A����������Ӧ����43.8 kJ��������
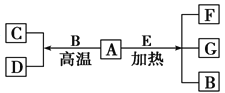
��1��д��AԪ�ص�����________��
��2������֪��
A(s)+O2(g)=G(g)��H= ��393.6 kJmol-1
C(g)+ O2(g)=G(g)��H=��283 kJmol-1
D(g)+ O2(g)=B(g)��H=��242 kJmol-1�ɴ��жϡ���Ϊ283 kJmol-1+242 kJmol-1>393.6 kJmol-1������Aȼ��ʱ������B���Էų����������������˵���Ƿ���ȷ��___________��������_____________________��
��3��д��A+O2��C���Ȼ�ѧ����ʽ��__________________________��
���𰸡� ̼ ����ȷ 1 mol A��O2ֱ��ȼ�շų�������Ϊ393.6 kJ����1 mol A����B��Ӧ����C ��D��C��D����O2��Ӧ����������-131.4 kJ+283 kJ+242 kJ=393.6 kJ��������ͬ C(s)+1/2O2(g)====CO(g) ��H=��110.6 kJmol-1
��������A��DΪ���ʣ�G�Ǻ�AԪ�ص��������壬��Ҫ��֪AΪ�ǽ���Ԫ�أ�A��DΪ���ʣ���֪A��B�������û���Ӧ����A��C������������Ӧ���ɺ�A���������壬���ϴ���������ѧ������Ԫ����̼��������A��B�û��IJ���DҲ���Ժ�������Ӧ������Aֻ��Ϊ̼Ԫ�أ�BΪˮ��CΪһ����̼��DΪ������GΪ������̼��
��1��AΪ̼Ԫ�أ��ʴ�Ϊ��̼��
��2����Ϊ1molA��O2ֱ��ȼ�շų�������Ϊ393.6kJ����1molA����B��Ӧ����C��D��C��D����O2��Ӧ�����ų�������-131.4kJ+283kJ+242kJ=393.6kJ��������ͬ��������Ŀ�е�˵������ȷ���ʴ�Ϊ������ȷ����Ϊ1molA��O2ֱ��ȼ�շų�������Ϊ393.6kJ����1molA����B��Ӧ����C��D��C��D����O2��Ӧ�����ų�������-131.4kJ+283kJ+242kJ=393.6kJ��������ͬ��
��3��C��s��+![]() O2��g���TCO��g�����Կ����Ƿ�Ӧ��A��s��+O2��g���TG��g����H=-393.6kJmol-1��ȥ��Ӧ��C��g��+
O2��g���TCO��g�����Կ����Ƿ�Ӧ��A��s��+O2��g���TG��g����H=-393.6kJmol-1��ȥ��Ӧ��C��g��+![]() O2��g���TG��g����H=-283kJmol-1�õ������ݸ�˹���ɿ�֪����H�T-393.6kJmol-1-��-283kJmol-1��=-110.6kJmol-1�����Ȼ�ѧ����ʽΪ��C��s��+
O2��g���TG��g����H=-283kJmol-1�õ������ݸ�˹���ɿ�֪����H�T-393.6kJmol-1-��-283kJmol-1��=-110.6kJmol-1�����Ȼ�ѧ����ʽΪ��C��s��+![]() O2��g���TCO��g����H=-110.6kJmol-1���ʴ�Ϊ��C��s��+
O2��g���TCO��g����H=-110.6kJmol-1���ʴ�Ϊ��C��s��+![]() O2��g���TCO��g����H=-110.6kJmol-1��
O2��g���TCO��g����H=-110.6kJmol-1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������У����������������ӵĺ�����Ӳ�����ȵ��ǣ� ��
A.Mg2+
B.Al3+
C.Be2+
D.H+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڷ�ӦKClO3��6HCl(Ũ)===3Cl2����KCl��3H2O��������ԭ����ԭ�Ӻͱ���������ԭ�ӵĸ���֮��Ϊ
A��1:6 B��5:1 C��1:5 D��6:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����25����101 kPa�����£�C(s)��H2(g)��CH3COOH(l)��ȼ���ȷֱ�Ϊ393.5kJ/mol��285.8kJ/mol��870.3kJ/mol����2C(s)��2H2(g)��O2(g)=CH3COOH(l)�ķ�Ӧ��Ϊ (����)
A����488.3 kJ/mol B����488.3 kJ/mol
C����191 kJ/mol D����191 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѧ����ѡ��3�����ʽṹ�����ʡ�
��һ����Ԫ�أ�selenium���ǵ������ڡ���VIA��Ԫ�أ������к����������ͻ��������л��������õĵ��ȵ����ԣ����������Ĺ��ЧӦ����Ӧ���ڹ��뵼����ϡ���ش��������⣺
��1��Seԭ�ӵĺ�������Ų�ʽΪ[Ar]_____��
��2��As��Se��ͬһ���ڵ�Ԫ�أ�As�ĵ�һ�����ܱ�Se��ԭ����______��SeO42-��Se�DO�ļ��DZ�SeO3�ļ���________�����С������
��3��H2Se��H2Sˮ��Һ������ǿ��ԭ����_____��____��
��4�������ľ���Ϊ���������ṹ��ԭ������Ϊ�������������ֲ������������ϣ�ͬһ������ԭ�����ú�ǿ��������֮��ԭ�����ý�������������״ͼ�������ṹͼ�;�������ͼ���¡�

��������Seԭ�ӵ��ӻ���ʽΪ____________��
����������CuSO4��NaOH�Ʊ���Cu��OH��2����ȩ��ʱ�����ɺ�ɫCu2O���侧���ṹ��ͼ��ʾ��

�ٸþ���ԭ���������AΪ��0��0��0����BΪ��1��0��0����CΪ��1/2��1/2��1/2������Dԭ�ӵ��������Ϊ_________��������ͭԭ�ӡ�
����Cu2O�����ܶ�Ϊd g��cm��3����������Ϊa pm�����ӵ�����ֵNA=________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��X��Y��Z��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������Wԭ�ӵ���������������������������������˵������ȷ���ǣ� ��

A. ԭ�Ӱ뾶��W��Z��Y��X
B. ����������Ӧˮ��������ԣ�X��W��Z
C. �����̬�⻯������ȶ��ԣ�Y��X��W��Z
D. Ԫ��X��Z��W������ϼ۷ֱ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʰ��մ������������ʺͷǵ����˳�����е��ǣ� ��
A. ���ᡢ��Ȼ�������ᡢ�ɱ� B. ������Ư�ۡ��Ȼ��ء�����
C. �Ȼ��ơ��������֡������ơ��Ҵ� D. ����������������������Լء�ʯ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����������������뵪ѭ�����ϳɰ���ҵ���Dz�����ֶ�֮һ������Ȼ��Ϊԭ�Ϻϳɰ����µ��������ʵķ�������������ȾС���ɱ��͵�����ŵ�������̴�����ͼ1��ʾ��

����д������Ȼ���Ʊ������Ļ�ѧ����ʽ��_________________________________��
��д���ϳ�����[CO (NH2) 2]��Ӧ�Ļ�ѧ����ʽ��__________________________��
��д��O2��NH3���д�������������Ӧ����NH4NO3��H2O�Ļ�ѧ����ʽ��______��
��ÿ����1molNH4NO3������ҪNH3______mol����Ҫ������ЩNH3��������ҪCH4____mol��
��2����ѧ���ѻ�ü��������о������N4���ӣ���ṹΪ�������壨��ͼ2������֪����1molN-N������193kJ����������1molN��N������941kJ��������1molN4����ת��ΪN2ʱҪ�ͷ�______kJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ���ǣ� ��
A.���ӻ������п��Ժ��й��ۼ��������ۻ�������һ���������Ӽ�
B.���й��ۼ�������һ���ǹ��ۻ�����
C.HCl����ˮֻ��˷����Ӽ�������
D.�ɱ�����ʱ�������ڹ��ۼ��ᷢ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com