
����Ŀ����¯���������з�����Ӧ�� ![]() Fe2O3(s)��CO(g)
Fe2O3(s)��CO(g)![]()
![]() Fe(s)��CO2(g)���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����ұ�������˵����ȷ���ǣ� ��
Fe(s)��CO2(g)���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����ұ�������˵����ȷ���ǣ� ��
�¶�T/�� | 1000 | 1150 | 1300 |
ƽ�ⳣ��K | 4.0 | 3.7 | 3.5 |
A. �ɱ������ݿ��жϸ÷�Ӧ����Ӧ������������������������
B. 1000����Fe2O3��CO��Ӧ��t min�ﵽƽ��ʱc(CO) =2��10-3 mol/L������CO2��ʾ�÷�Ӧ��ƽ������Ϊ2��10��3/t mol��L��1��min��1
C. Ϊ��ʹ�÷�Ӧ��K��������������������ʱ������c(CO)
D. ������������ʱ������Fe2O3��������������Ч��������β����CO�ĺ���
���𰸡�D
��������
A���ɱ������ݿ��жϣ�ƽ�ⳣ�����¶�����С��˵����ӦΪ���ȷ�Ӧ���÷�Ӧ����Ӧ����������������������������A����B��1000����Fe2O3��CO��Ӧ��tmin�ﵽƽ��ʱc��CO��=2��10-3mol/L����CO��ʼŨ��x��
![]() Fe2O3��s��+CO��g��
Fe2O3��s��+CO��g��![]() Fe��s��+CO2��g����
Fe��s��+CO2��g����
��ʼ����mol/L��x 0
�仯����mol/L��x-2��10-3 x-2��10-3
ƽ������mol/L��2��10-3 x-2��10-3
K=![]() =4��x=10-2mol/L����CO��ʾ�÷�Ӧ��ƽ������Ϊ
=4��x=10-2mol/L����CO��ʾ�÷�Ӧ��ƽ������Ϊ![]() ����B����C��ƽ�ⳣ��ֻ���¶�Ӱ�죬����c��CO�����ܸı仯ѧƽ�ⳣ������C����D�����������������ı�ƽ����ƶ�����������������ʱ������Fe2O3��������������Ч��������β����CO�ĺ�������D��ȷ����ΪD��
����B����C��ƽ�ⳣ��ֻ���¶�Ӱ�죬����c��CO�����ܸı仯ѧƽ�ⳣ������C����D�����������������ı�ƽ����ƶ�����������������ʱ������Fe2O3��������������Ч��������β����CO�ĺ�������D��ȷ����ΪD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������������Ź㷺����;����ش��������⡣
(1) Fe2+�����������Ų�ʽ____________��Ԫ��Fe��Mn�ĵ��������ֱܷ�ΪI3(Fe)��I3(Mn)����I3(Fe)______I3(Mn)(����>������<")��
(2)��̬�Ȼ����ķ������Ϊ(AlCl3)2������Al��Cl����8e-�ȶ��ṹ��Alԭ�ӵ��ӻ���ʽΪ__________�����ݵȵ���ԭ����AlO2-�Ŀռ乹��Ϊ_____��
(3) Fe(CO)5���۵�Ϊ-20 �����е�Ϊ103 �������������ѣ��侧������Ϊ______��
(4) ��ѧ���Ƿ���ijЩ���������ʿɴ����غϳ���(N2H4)���е㣺N2H4>C2H6����Ҫԭ��Ϊ______________________��
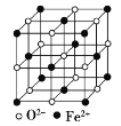
(5) FeO����ľ�����ͼ��ʾ����֪��FeO������ܶ�Ϊ�� g/cm3��NA���������ӵ�������ֵ���ڸþ����У���Fe2+�����ҵȾ����Fe2+��ĿΪ_____��Fe2+��O2-��̺˼��Ϊ______pm(������NA��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����̬Cuԭ�ӵĺ�������Ų�ʽΪ____________________
��2����ԭ�ӹ���ص���ʽ���ǣ��������еĹ��ۼ�������____________��
��3��![]() ˮ��Һ��
ˮ��Һ��
��ˮ��������ԭ�ӵ��ӻ�������_____��![]() ������____
������____![]() ����
����![]() ����
����![]() ������
������![]() ��
��![]()
�ڲ����ڵ�������������______ ��
A.���Ӽ� ![]() ���Լ�
���Լ� ![]() ��λ��
��� ![]() ���
��� ![]() ���»���
���»���
��4����ͭ��ұ��ͭʱ������![]() �ɾ���
�ɾ���![]() ;���γ����ꡣ
;���γ����ꡣ
��![]() �Ŀռ乹��Ϊ________���ӽṹ�Ƕȣ�����
�Ŀռ乹��Ϊ________���ӽṹ�Ƕȣ�����![]() ������ǿ��
������ǿ��![]() ��ԭ����_______
��ԭ����_______
����֪����ԭ�ӷ����У���ԭ�Ӷ���ͬһƽ��������Щԭ�����ƽ�е�p�������p���ӿ��ڶ��ԭ�Ӽ��˶����γ�������![]() ����
����![]() ���
���![]() ��
��![]() ����
����![]() ������
������![]() ��ʾ������m��n�ֱ���������γɴ�
��ʾ������m��n�ֱ���������γɴ�![]() ����ԭ�Ӹ����͵��������籽�����д�
����ԭ�Ӹ����͵��������籽�����д�![]() ����ʾΪ
����ʾΪ![]() ���������д���������
�����������������![]() ��������_____��
��������_____��
A.![]() B.
B.![]() C.
C.![]()
��ͭ������Cuԭ�ӵĶѻ���ʽ��ͼ����ʾ����ѻ���ʽΪ_____����λ��Ϊ_______��

�ܽ�ͭ�Ͻ�ľ�����ͼ����ʾ����ͭ�Ͻ���д���ܣ������Auԭ��λ�ڶ��㣬Cuԭ��λ�����ģ�Hԭ���������1��Auԭ�Ӻ;�Auԭ�������3��Cuԭ�ӹ��ɵ��������϶�У���Cuԭ����Auԭ�ӵ���̾���Ϊ![]() �������ӵ�������λΪ
�������ӵ�������λΪ![]() ����þ��崢����ܶ�Ϊ______
����þ��崢����ܶ�Ϊ______![]() �г�����ʽ
�г�����ʽ![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ���κ���úȼ�ղ����Ĵ�����Ⱦ��ij����������µ����۷���������������ͼ��ʾ����������������ǣ� ��
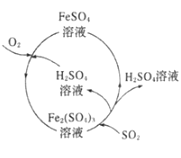
A.�����̿��Գ�ȥúȼ��ʱ����β���е�SO2�����Ϊ��
B.��������H2SO4��Fe2��SO4��3��Һ����ѭ������
C.������ÿ����11.2LSO2����״����ͬʱ����1molFe2+
D.�������漰��Fe2+�������ķ�Ӧ�����ӷ���ʽΪ4Fe2++O2+2H2O�T4Fe3++4OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ϊmg��ͭм��ȫ��������Ũ�����У���Ӧ��õ�NO2��NO�Ļ�����壬����������ͨ��300mL2molL-1NaOH��Һ�У�ǡ����ȫ��Ӧ�����ɺ�NaNO3��NaNO2������Һ������NaNO3�����ʵ���Ϊ0.2mol����m��ֵΪ�� ��
A.12.8B.19.2C.25.6D.51.2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ�ת���У�A��X�dz������ʣ�Y�ڳ�����Ϊ��ɫҺ�壬B��C��D��Ϊ��ѧ��ѧ�г����Ļ��������֮���ת����ϵ��ͼ��ʾ�����ֲ���ͷ�Ӧ��������ȥ����
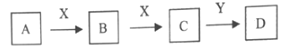
�ش��������⣺
��1����BΪ�ܵ�����������壬��D�Ļ�ѧʽ������__��
��2����CΪ����ɫ���壬DΪǿ����Һ����C��D�����ӷ���ʽΪ__��
��3����AΪ����ɫ���壬DΪǿ�ᣬ��D��Ũ��Һ��̼��Ӧ��������B�Ļ�ѧ����ʽΪ__��
��4����AΪ����ɫ���壬XΪ������������X��B��Һ��Ӧ�����ӷ���ʽΪ__�������Լ��ܼ���B��Һ��C��Һ����__�������ţ�
A.AgNO3��Һ B.KSCN��Һ C.���� D.NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
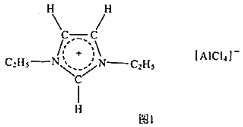
����Ŀ������Һ����һ��ֻ��������ɵ�Һ�壬�ڵ�����Ҳ����Һ̬�ȶ����ڣ���һ�ֺ����о���ֵ���ܼ���������Һ����о���ʾ���������Һ����Ҫ�����µ������Ӻ�������ɣ�
�ش��������⣺
��1��![]() �����ڱ��е�λ����______����۵����Ų�ʽΪ______ͼ1�и����ӵĿռ乹��Ϊ______��
�����ڱ��е�λ����______����۵����Ų�ʽΪ______ͼ1�и����ӵĿռ乹��Ϊ______��
��2���Ȼ������۵�Ϊ![]() �����������۵�ߴ�
�����������۵�ߴ�![]() �����Ƕ��ǻ��ý����ͷǽ����Ļ�����۵������ô���ԭ����______��
�����Ƕ��ǻ��ý����ͷǽ����Ļ�����۵������ô���ԭ����______��
��3��ͼ�������������˾�����ȶ��ԣ����ĵ������价״�ṹ�и߶�������������Nԭ�ӵ��ӻ���ʽΪ______��Cԭ�ӵ��ӻ���ʽΪ______��
��4��Ϊ��ʹ�������Ե�����ʽ�����Ի�����õ��ܽ����ܣ���Nԭ��������![]() ���ܱ�Hԭ���滻�������ԭ��______��
���ܱ�Hԭ���滻�������ԭ��______��
��5��![]() ��Mg��Al����Ԫ���ĵ�һ�������ɴ�С��˳����______��
��Mg��Al����Ԫ���ĵ�һ�������ɴ�С��˳����______��
��6����֪�������ľ����ṹ��ͼ2��ʾ�������е�ԭ�Ӷѻ���ʽ��ͼ3��ʾ�����ֶѻ���ʽ��Ϊ______���������ױ߱߳�Ϊacm����Ϊccm�������ӵ�������ֵΪN��������������ܶ�Ϊ______![]() �г�����ʽ
�г�����ʽ![]() ��
��
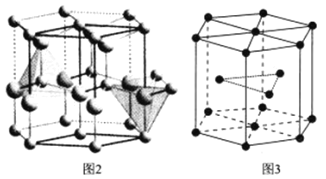
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܱ������з�����ӦX(g)��3Y(g)![]() 2Z(g)����X��Y��Z����ʼŨ�ȷֱ�Ϊ0.1 mol��L��1��0.3mol��L��1��0.2mol��L��1����ƽ��ʱ�����ʵ�Ũ�Ȳ������ǣ� ��
2Z(g)����X��Y��Z����ʼŨ�ȷֱ�Ϊ0.1 mol��L��1��0.3mol��L��1��0.2mol��L��1����ƽ��ʱ�����ʵ�Ũ�Ȳ������ǣ� ��
A. XΪ0.2 mol��L��1
B. YΪ0.1 mol��L��1
C. ZΪ0.3 mol��L��1
D. ZΪ0.1 mol��L��1ʱ��YΪ0.45 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯�����������������д��ڹ㷺��;��

��1����ͼ����Ԫ����Ԫ�����ڱ��е��й���Ϣ��
��ȫ��ԭ�ӵĺ�������Ų�ʽ_____________3p63d64s2����55.85����________________________����Ȼ���д��ڵ�54Fe��56Fe�����ǻ���Ϊ_____________��
��2������Ƭ������Ũ�����У�Ƭ�̺���Ƭ��������ͭ��Һ�У�������Ƭ���������Ա仯��ԭ����_____________��ͨ��֤��ij��Һ�к�Fe2+�Ļ�ѧ������_____________________��
��3��SO2 ��ǿ��ԭ�ԣ�д������ FeCl3 ��Һ��Ӧ�����ӷ���ʽ______________________��
���� 0.4mol FeCl3 ������Ӧ������Ҫ��״���µ� SO2______________����
��4�����Ƶ��ˮͨ���� Fe3+������ˮ�м��� Na2CO3 ��Һ����������ɫ��������ɫ��ζ�����塣���ƽ���ƶ��ǶȽ�����һ����___________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com