
ΓΨΧβΡΩΓΩ¬ΝΓΔΧζ‘Ύ…ζΜνΓΔ…ζ≤ζ÷–”–Ή≈ΙψΖΚΒΡ”ΟΆΨΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΓΘ
(1) Fe2+ΒΡΉνΆβ≤ψΒγΉ”≈≈≤Φ Ϋ____________ΓΘ‘ΣΥΊFe”κMnΒΡΒΎ»ΐΒγάκΡήΖ÷±πΈΣI3(Fe)ΓΔI3(Mn)Θ§‘ρI3(Fe)______I3(Mn)(ΧνΓΑ>Γ±ΓΔΓΑ<")ΓΘ
(2)ΤχΧ§¬»Μ·¬ΝΒΡΖ÷Ή”Ήι≥…ΈΣ(AlCl3)2Θ§Τδ÷–AlΓΔClΨυ¥ο8e-Έ»Ε®ΫαΙΙΘ§Al‘≠Ή”ΒΡ‘”Μ·ΖΫ ΫΈΣ__________ΓΘΗυΨίΒ»ΒγΉ”‘≠άμΘ§AlO2-ΒΡΩ’ΦδΙΙ–ΆΈΣ_____ΓΘ
(3) Fe(CO)5ΒΡ»έΒψΈΣ-20 ΓφΘ§Ζ–ΒψΈΣ103 ΓφΘ§“Ή»ή”Ύ““Ο―Θ§ΤδΨßΧεάύ–ΆΈΣ______Θ§
(4) ΩΤ―ßΦ“Ο«ΖΔœ÷Ρ≥–©Κ§ΧζΒΡΈο÷ Ω…¥ΏΜ·ΡρΥΊΚœ≥…κ¬(N2H4)Θ§Ζ–ΒψΘΚN2H4>C2H6ΒΡ÷ς“Σ‘≠“ρΈΣ______________________ΓΘ
(5) FeOΨßΧεΒΡΨßΑϊ»γΆΦΥυ ΨΘ§ΦΚ÷ΣΘΚFeOΨßΧεΒΡΟήΕ»ΈΣΠ― g/cm3Θ§NA¥ζ±μΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷ΒΓΘ‘ΎΗΟΨßΑϊ÷–Θ§”κFe2+ΫτΝΎ«“Β»ΨύάκΒΡFe2+ ΐΡΩΈΣ_____ΘΜFe2+”κO2-ΉνΕΧΚΥΦδΨύΈΣ______pm(”ΟΠ―ΚΆNA±μ Ψ)ΓΘ
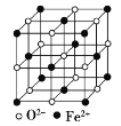
ΓΨ¥πΑΗΓΩ3s23p63d6 ΘΦ sp3 ÷±œΏ–Έ Ζ÷Ή”ΨßΧε «Α’ΏΩ…–Έ≥…Ζ÷Ή”Φδ«βΦϋΘ§Κσ’Ώ÷Μ”–ΖΕΒ¬ΜΣΝΠ 12 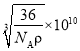
ΓΨΫβΈωΓΩ
Θ®1Θ©ΚΥΆβΒγΉ”≈≈≤ΦΑ¥ΡήΦΕΥ≥–ρ≈≈≤ΦΘ§‘ΎΜ·―ßΖ¥”Π÷– ß»ΞΒγΉ”Α¥¥”ΉνΆβ≤ψΒΫ¥ΈΆβ≤ψ“ά¥Έ ß»ΞΘΜΗυΨίΦέΒγΉ”≈≈≤Φ ΫΖ÷ΈωΒψΒγάκΡήΒΡ¥σ–ΓΘΜ
Θ®2Θ©ΗυΨίΠ“ΦϋΒγΉ”Ε‘ ΐΚΆΙ¬ΒγΉ”Ε‘ ΐ≈–Εœ‘”Μ·ΖΫ ΫΘΜ”ΟΧφ¥ζΖ®Ζ÷ΈωΒ»ΒγΉ”ΧεΘΜ
Θ®3Θ©ΫαΚœΨßΧεάύ–Ά”κ–‘÷ ΒΡΙΊœΒΉς¥πΘΜ
Θ®4Θ©ΗυΨίΨßΧεάύ–ΆΚΆΖ–ΒψΗΏΒΆΒΡ±»ΫœΉς¥πΘΜ
Θ®5Θ©ΗυΨίΨßΑϊ Ψ“βΆΦΩ…÷ΣΘ§―«ΧζάκΉ”ΈΜ”ΎΨßΑϊΒΡΕΞΒψΚΆΟφ–ΡΘ§O2-άκΉ”ΈΜ”ΎΨßΑϊΒΡάβ…œΚΆΧε–ΡΘ§ΫαΚœΠ―=![]() ΦΤΥψΓΘ
ΦΤΥψΓΘ
Θ®1Θ©ΜυΧ§Fe‘≠Ή”ΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ[Ar]3d64s2Θ§‘≠Ή” ß»ΞΒγΉ”¥”ΉνΆβ≤ψΒΫ¥ΈΆβ≤ψ“ά¥Έ ß»ΞΘ§‘ρFe2+ΒΡΉνΆβ≤ψΒγΉ”≈≈≤Φ ΫΈΣ3s23p63d6ΘΜΧζ‘ΣΥΊ ß»ΞΒΡΒΎ»ΐΗωΒγΉ” «3d6…œΒΡΒγΉ”Θ§Εχ3d6»ί“Ή ß»Ξ“ΜΗωΒγΉ”–Έ≥…±»ΫœΈ»Ε®ΒΡ3d5Ακ¬ζΉ¥Χ§Θ§ΕχMnΒΡΦέΒγΉ”≈≈≤Φ ΫΈΣ3d53s2Θ§ ß»ΞΒΡΒΎ»ΐΗωΒγΉ” «3d5…œΒΡΒγΉ”Θ§’β «±»ΫœΈ»Ε®ΒΡΑκ≥δ¬ζΉ¥Χ§Θ§Υυ“‘Ρ― ß»ΞΘ§Ι I3(Fe) ΘΦI3(Mn)ΓΘ
Θ®2Θ©ΗυΨίΤχΧ§¬»Μ·¬ΝΒΡΖ÷Ή”Ήι≥…ΈΣ(AlCl3)2Θ§“Σ ΙΟΩΗω‘≠Ή”ΕΦ¥οΒΫ8e-Έ»Ε®ΫαΙΙΘ§Al≥ΐΝΥ”κ»ΐΗωCl–Έ≥…»ΐΗωΙ≤ΦέΒΞΦϋΆβΘ§ΜΙ“Σ”…ClΧαΙ©“ΜΗω≈δΈΜΦϋΘ§Υυ“‘–η“Σ≤…»Γsp3‘”Μ·ΖΫ Ϋ–Έ≥…ΥΡΗωΒ»ΦέΒΡΙ≤ΦέΦϋΘΜAlO2-÷–Κ§”–16ΗωΦέΒγΉ”ΓΔ3Ηω‘≠Ή”Θ§”κCO2ΥυΚ§ΒΡ‘≠Ή”Ήή ΐΓΔΦέΒγΉ”Ήή ΐΕΦœύΆ§Θ§CO2ΒΡΩ’ΦδΙΙ–ΆΈΣ÷±œΏ–ΈΘ§ΗυΨίΒ»ΒγΉ”‘≠άμΘ§AlO2-ΒΡΩ’ΦδΙΙ–ΆΈΣ÷±œΏ–ΈΓΘ
Θ®3Θ©ΗυΨίFe(CO)5»έΖ–ΒψΒΆΓΔ“Ή»ή”Ύ”–Μζ»ήΦΝΒΡΈοάμ–‘÷ Θ§Ω…≈–ΕœΤδΈΣΖ÷Ή”ΨßΧεΓΘ
Θ®4Θ©‘ΎN2H4Ζ÷Ή”÷–¥φ‘ΎNΓΣHΦϋΘ§Υυ“‘ΡήΙΜ‘ΎΖ÷Ή”Φδ–Έ≥…«βΦϋΘ§ΕχC2H6Ζ÷Ή”÷Μ”–ΖΕΒ¬ΜΣΝΠΘ§Ι «Α’ΏΖ–ΒψΗΏ”ΎΚσ’ΏΓΘ
Θ®5Θ©”…ΗΟΨßΑϊΫαΙΙΩ…÷ΣΘ§Fe2+ΫτΝΎΒΡFe2+ΒΡΉνΕΧΨύάκΈΣΟφΕ‘Ϋ«œΏΒΡ![]() Θ§“‘Οφ–ΡFe2+ΈΣ―–ΨΩΕ‘œσΘ§”κFe2+ΫτΝΎ«“Β»ΨύάκΒΡFe2+ ΐΡΩΈΣ12ΗωΘΜΗΟΨßΧε÷–Κ§”–ΒΡFe2+ΒΡΗω ΐΈΣ
Θ§“‘Οφ–ΡFe2+ΈΣ―–ΨΩΕ‘œσΘ§”κFe2+ΫτΝΎ«“Β»ΨύάκΒΡFe2+ ΐΡΩΈΣ12ΗωΘΜΗΟΨßΧε÷–Κ§”–ΒΡFe2+ΒΡΗω ΐΈΣ![]() Θ§O2-ΒΡΗω ΐΈΣ
Θ§O2-ΒΡΗω ΐΈΣ![]() Θ§Φ¥Κ§”–4ΗωΓΑFeOΓ±Θ§Υυ“‘ΗΟΨßΑϊΒΡ÷ ΝΩΈΣ
Θ§Φ¥Κ§”–4ΗωΓΑFeOΓ±Θ§Υυ“‘ΗΟΨßΑϊΒΡ÷ ΝΩΈΣ![]() Θ§‘ρΨßΑϊΒΡ±Ώ≥ΛΈΣ
Θ§‘ρΨßΑϊΒΡ±Ώ≥ΛΈΣ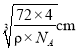 Θ§ΕχFe2+”κO2-ΉνΕΧΚΥΦδΨύΈΣ±Ώ≥ΛΒΡ“ΜΑκΘ§Φ¥
Θ§ΕχFe2+”κO2-ΉνΕΧΚΥΦδΨύΈΣ±Ώ≥ΛΒΡ“ΜΑκΘ§Φ¥ ΓΘ
ΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ Β―ι “”ΟΦ”»»1“ΜΕΓ¥ΦΓΔ≈®H2SO4ΚΆδεΜ·ΡΤΜλΚœΈοΒΡΖΫΖ®ά¥÷Τ±Η1“ΜδεΕΓΆιΘ§…ηΦΤΝΥ»γΆΦΥυ ΨΒΡ Β―ιΉΑ÷Ο![]() Τδ÷–ΒΡΦ–≥÷“«Τς“― Γ¬‘
Τδ÷–ΒΡΦ–≥÷“«Τς“― Γ¬‘![]() ΓΘ
ΓΘ
“―÷ΣΘΚH2SO4ΘΪNaBr=NaHSO4ΘΪHBrΘ§ H2SO4Θ®≈®Θ©ΘΪ2HBr=Br2ΘΪSO2ΓϋΘΪ2H2O
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)“«ΤςaΒΡΟϊ≥ΤΈΣ______ΓΘ
(2)÷Τ±Η≤ΌΉς÷–Θ§Φ”»κΒΡ≈®ΝρΥα ¬œ»“ΣΫχ––œΓ ΆΘ§ΤδΡΩΒΡ «______![]() Χν―ΓœνΉ÷ΡΗ
Χν―ΓœνΉ÷ΡΗ![]() ΓΘ
ΓΘ
![]() Φθ…ΌΗ±≤ζΈοœ©ΚΆΟ―ΒΡ…ζ≥…
Φθ…ΌΗ±≤ζΈοœ©ΚΆΟ―ΒΡ…ζ≥…![]() Φθ…Ό
Φθ…Ό![]() ΒΡ…ζ≥…
ΒΡ…ζ≥…![]() Υ° «Ζ¥”ΠΒΡ¥ΏΜ·ΦΝ
Υ° «Ζ¥”ΠΒΡ¥ΏΜ·ΦΝ
(3)–¥≥ω¥Υ Β―ι÷Τ1“ΜδεΕΓΆιΒΡΉήΜ·―ßΖΫ≥Χ Ϋ______ΓΘ
(4)”–Ά§―ßΡβΆ®ΙΐΚλΆβΙβΤΉ“«ΦχΕ®ΥυΒΟ≤ζΈο÷– «ΖώΚ§”–ΓΑ![]() Γ±Θ§ά¥»ΖΕ®Η±≤ζΈο÷– «Ζώ¥φ‘ΎΕΓΟ―
Γ±Θ§ά¥»ΖΕ®Η±≤ζΈο÷– «Ζώ¥φ‘ΎΕΓΟ―![]() «κΤάΦέΗΟΆ§―ß…ηΦΤΒΡΦχΕ®ΖΫΑΗ «ΖώΚœάμΘΩάμ”… «______ΓΘ
«κΤάΦέΗΟΆ§―ß…ηΦΤΒΡΦχΕ®ΖΫΑΗ «ΖώΚœάμΘΩάμ”… «______ΓΘ
(5)ΈΣΝΥΫχ“Μ≤ΫΧα¥Ω1“ΜδεΕΓΆιΘ§ΗΟ–ΓΉιΆ§―ß≤ιΒΟœύΙΊ”–ΜζΈοΒΡ”–ΙΊ ΐΨί»γ±μΘΚ
Έο÷ | »έΒψ | Ζ–Βψ |
1“ΜΕΓ¥Φ |
|
|
1“ΜδεΕΓΆι |
|
|
ΕΓΟ― |
|
|
1“ΜΕΓœ© |
|
|
‘ρ”ΟBΉΑ÷ΟΆξ≥…¥ΥΧα¥Ω Β―ι ±ΘΜΘ§ Β―ι÷–“Σ―ΗΥΌ…ΐΗΏΈ¬Ε»÷Ν______ ’Φ·ΥυΒΟΝσΖ÷ΓΘ
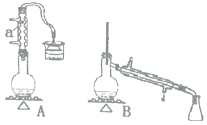
(6)»τ Β―ι÷–Υυ»Γ1“ΜΕΓ¥ΦΓΔNaBrΖ÷±πΈΣ![]() ΓΔ
ΓΔ![]() Θ§≈®ΝρΥα
Θ§≈®ΝρΥα![]() Θ§’τ≥ωΒΡ¥÷≤ζΈοΨ≠œ¥Β”Θ§Η…‘οΚσ‘Ό¥Έ’τΝσΒΟΒΫ
Θ§’τ≥ωΒΡ¥÷≤ζΈοΨ≠œ¥Β”Θ§Η…‘οΚσ‘Ό¥Έ’τΝσΒΟΒΫ![]() “ΜδεΕΓΆιΘ§‘ρ1“ΜδεΕΓΆιΒΡ≤ζ¬ «______
“ΜδεΕΓΆιΘ§‘ρ1“ΜδεΕΓΆιΒΡ≤ζ¬ «______![]() ±ΘΝτ2ΈΜ”––ß ΐΉ÷
±ΘΝτ2ΈΜ”––ß ΐΉ÷![]() ΓΘ
ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΗυΨί‘”Μ·ΙλΒάάμ¬έΚΆΦέΒγΉ”Ε‘ΜΞ≥βάμ¬έΡΘ–Ά≈–ΕœΘ§œ¬Ν–Ζ÷Ή”ΜράκΉ”ΒΡ÷––Ρ‘≠Ή”ΒΡ‘”Μ·ΖΫ ΫΦΑΩ’ΦδΙΙ–Ά’ΐ»ΖΒΡ «Θ® Θ©
―Γœν | Ζ÷Ή”ΜράκΉ” | ÷––Ρ‘≠Ή”‘”Μ·ΖΫ Ϋ | ΦέΒγΉ”Ε‘ΜΞ≥βάμ¬έΡΘ–Ά | Ζ÷Ή”ΜράκΉ”ΒΡΩ’ΦδΙΙ–Ά |
A |
|
| ÷±œΏ–Έ | ÷±œΏ–Έ |
B |
|
| ΤΫΟφ»ΐΫ«–Έ | »ΐΫ«ΉΕ–Έ |
C |
|
| ΥΡΟφΧε–Έ | ΤΫΟφ»ΐΫ«–Έ |
D |
|
| ΥΡΟφΧε–Έ | ’ΐΥΡΟφΧε–Έ |
A.AB.BC.CD.D
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ≥―–ΨΩ–‘―ßœΑ–ΓΉι”Ο»γΆΦΉΑ÷ΟΫχ––SO2”κFeCl3»ή“ΚΖ¥”ΠΒΡœύΙΊ Β―ιΘΚ
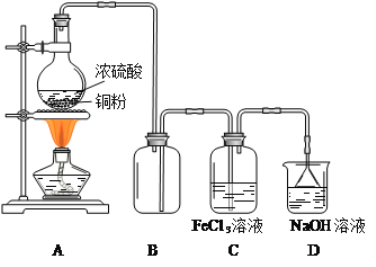
Άξ≥…œ¬Ν–ΧνΩ’
Θ®1Θ©‘Ύ≈δ÷ΤFeCl3»ή“Κ ±Θ§–ηœ»Α―FeCl3ΨßΧε»ήΫβ‘Ύ________÷–Θ§‘ΌΦ”Υ°œΓ ΆΘ§≤≈ΡήΒΟΒΫΆΗΟς≥Έ«ε»ή“ΚΓΘ
Θ®2Θ©BΉΑ÷ΟΒΡΉς”Ο «__________ΘΜDΉΑ÷Ο÷–Έϋ ’Έ≤ΤχΒΡάκΉ”ΖΫ≥Χ Ϋ «__________ΓΘ
Θ®3Θ©AΉΑ÷Ο÷–÷Τ±ΗSO2ΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ_______Θ§‘ΎΗΟΖ¥”Π÷–≈®ΝρΥαΧεœ÷ΒΡ–‘÷ «______ΓΘ
Θ®4Θ©Β±Έ¬Ε»ΫœΗΏΒΡSO2ΤχΧεΘ®ΉψΝΩΘ©Ά®»κFeCl3»ή“Κ÷– ±Θ§Ω…“‘Ιέ≤λΒΫ»ή“Κ”…ΜΤ…Ϊ÷πΫΞ±δΈΣ«≥¬Χ…ΪΓΘΗυΨί¥Υœ÷œσΘ§ΗΟ–ΓΉιΆ§―ß»œΈΣSO2”κFeCl3»ή“ΚΖΔ…ζΝΥ―θΜ·ΜΙ‘≠Ζ¥”ΠΓΘ
a.–¥≥ωSO2”κFeCl3»ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ___________ΓΘ
b.«κ…ηΦΤ Β―ιΖΫΑΗΦλ―ι…œ ωΖ¥”Π÷–FeCl3 «ΖώΖ¥”ΠΆξ»ΪΘΚ_________________ΓΘ
c.»τœρΒΟΒΫΒΡ«≥¬Χ…Ϊ»ή“Κ÷–÷πΒΈΒΈΦ”NaOH»ή“ΚΘ§Ω…“‘Ιέ≤λΒΫΒΡœ÷œσ «ΘΚ________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬±μΈΣ‘ΣΥΊ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷Θ§ ΜΊ¥πœ¬Ν–Έ ΧβΓΘ
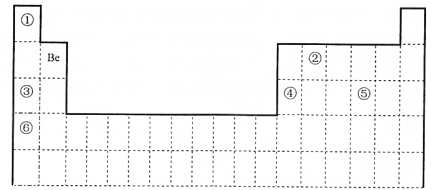
(1)Έ“ΙζΩΤ―ßΦ“≤…”ΟΓΑ¬Ννκ≤βΡξΖ®Γ±≤βΝΩΓΑ±±Ψ©»ΥΓ±ΡξΝδΓΘ10Be ΚΆ9Be______________
aΘ° «Ά§“Μ÷÷ΚΥΥΊ bΘ°ΨΏ”–œύΆ§ΒΡ÷–Ή” ΐ cΘ°ΜΞΈΣΆ§ΥΊ“λ–ΈΧε dΘ°ΜΞΈΣΆ§ΈΜΥΊ
(2)‘ΣΥΊΔΌΚΆΔΎΩ…–Έ≥…Εύ÷÷Μ·ΚœΈοΓΘœ¬Ν–ΡΘ–Ά±μ ΨΒΡΖ÷Ή”÷–Θ§≤ΜΩ…Ρή”…ΔΌΚΆΔΎ–Έ≥…ΒΡ «_______(Χν–ρΚ≈)ΓΘ
(3)‘ΣΥΊΔΌ~Δό÷–Θ§Ϋπ τ–‘Ήν«ΩΒΡ «___(Χν‘ΣΥΊΖϊΚ≈)ΓΘΔΎΒΡΒΞ÷ ΚΆΔίΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΒΡ≈®»ή“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______________ΓΘ
(4)Ββ(53I) «»ΥΧε±Ί–ηΒΡΈΔΝΩ‘ΣΥΊ÷°“ΜΓΘ
ΔΌΒβ(53I) ‘Ύ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο «______
ΔΎCI-ΓΔBr-ΓΔI-ΒΡΜΙ‘≠–‘”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ_______ΓΘ
ΔέΉ Νœœ‘ Ψ: Ag+ΚΆI-ΜαΖΔ…ζ―θΜ·ΜΙ‘≠Ζ¥”Π…ζ≥…ΝΫ÷÷ΒΞ÷ Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ_______ΓΘΡ≥Ά§―ß”ΟΆΦ Β―ι―ι÷Λ…œ ωΖ¥”ΠΘ§Ιέ≤λΒΫΝΫ»ή“ΚΜλΚœΚσΝΔΦ¥≥ωœ÷ΜΤ…ΪΜκΉ«Θ§‘ΌΦ”»κΒμΖέ»ή“ΚΘ§≤Μ±δάΕΓΘΖ÷Έω≤ζ…ζ…œ ωœ÷œσΒΡΩ…Ρή‘≠“ρ:________________
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ≥―–ΨΩ–ΓΉιΫχ––Mg(OH)2≥ΝΒμ»ήΫβΚΆ…ζ≥…ΒΡ Β―ιΧΫΨΩΓΘ
œρ2÷ß Δ”–1 mL 1 molΓΛL-1ΒΡMgCl2»ή“Κ÷–ΗςΦ”»κ10ΒΈ2 molΓΛL-1NaOHΘ§÷ΤΒΟΒ»ΝΩMg(OH)2≥ΝΒμΘΜ»ΜΚσΖ÷±πœρΤδ÷–Φ”»κ≤ΜΆ§ ‘ΦΝΘ§Φ«¬Φ Β―ιœ÷œσ»γœ¬±μΘΚ
Β―ι–ρΚ≈ | Φ”»κ ‘ΦΝ | Β―ιœ÷œσ |
Δώ | 4 mL 2 molΓΛL-1HCl »ή“Κ | ≥ΝΒμ»ήΫβ |
Δρ | 4 mL 2 molΓΛL-1NH4Cl »ή“Κ | ≥ΝΒμ»ήΫβ |
Θ®1Θ©¥”≥ΝΒμ»ήΫβΤΫΚβΒΡΫ«Ε»Ϋβ Ά Β―ιΔώΒΡΖ¥”ΠΙΐ≥Χ_____________ΓΘ
Θ®2Θ©≤βΒΟ Β―ιΔρ÷–Υυ”ΟNH4Cl»ή“Κœ‘Υα–‘Θ®pH‘ΦΈΣ4.5Θ©Θ§”ΟάκΉ”ΖΫ≥Χ ΫΫβ ΆΤδœ‘Υα–‘ΒΡ‘≠“ρ___________ΓΘ
Θ®3Θ©ΦΉΆ§―ß»œΈΣ”Π≤Ι≥δ“ΜΗω Β―ιΘΚœρΆ§―υΒΡMg(OH)2≥ΝΒμ÷–Φ”4 mL’τΝσΥ°Θ§Ιέ≤λΒΫ≥ΝΒμ≤Μ»ήΫβΓΘΗΟ Β―ιΒΡΡΩΒΡ «_________ΓΘ
Θ®4Θ©Ά§―ßΟ«≤¬≤β Β―ιΔρ÷–≥ΝΒμ»ήΫβΒΡ‘≠“ρ”–ΝΫ÷÷ΘΚ“Μ «NH4Cl»ή“Κœ‘Υα–‘Θ§»ή“Κ÷–ΒΡH+Ω…“‘ΫαΚœOH- Θ§ΫχΕχ Ι≥ΝΒμ»ήΫβΘΜΕΰ «____________ΓΘ
Θ®5Θ©““Ά§―ßΦΧ–χΫχ–– Β―ιΘΚœρ4 mL 2 molΓΛL-1 NH4Cl»ή“Κ÷–ΒΈΦ”2ΒΈ≈®Α±Υ°Θ§ΒΟΒΫpH‘ΦΈΣ8ΒΡΜλΚœ»ή“ΚΘ§œρΆ§―υΒΡMg(OH)2≥ΝΒμ÷–Φ”»κΗΟΜλΚœ»ή“ΚΘ§Ιέ≤λœ÷œσΓΘ
ΔΌ Β―ιΫαΙϊ÷ΛΟςΘ®4Θ©÷–ΒΡΒΎΕΰ÷÷≤¬≤β «≥…ΝΔΒΡΘ§““Ά§―ßΜώΒΟΒΡ Β―ιœ÷œσ «___________ΓΘ
Δέ““Ά§―ß’β―υ≈δ÷ΤΜλΚœ»ή“ΚΒΡάμ”… «___________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–»»Μ·―ßΖΫ≥Χ ΫΦΑ”–ΙΊ”Π”ΟΒΡ–π ω÷–Θ§’ΐ»ΖΒΡ «Θ® Θ©
A.»τ2H2(g)+O2(g)=2H2O(g) ΠΛH=Θ≠483.6kJ/molΘ§‘ρH2ΒΡ»Φ…’»»ΈΣ241.8 kJ/mol
B.“―÷Σ«ΩΥα”κ«ΩΦν‘ΎœΓ»ή“ΚάοΖ¥”ΠΒΡ÷–ΚΆ»»ΈΣ57.3 kJ/molΘ§‘ρH2SO4(aq)+ Ba(OH)2(aq)= BaSO4(s)+2H2O(l) ΠΛH =Θ≠114.6 kJ/mol
C.500ΓφΓΔ30MPaœ¬Θ§ΫΪ0.5mol N2ΚΆ1.5mol H2÷Ο”ΎΟή±’ΒΡ»ίΤς÷–≥δΖ÷Ζ¥”Π…ζ≥…NH3(g)Θ§Ζ≈»»19.3kJΘ§Τδ»»Μ·―ßΖΫ≥Χ ΫΈΣΘΚN2(g)+3H2(g) ![]() 2NH3(g) ΠΛH =Θ≠38.6 kJ/mol
2NH3(g) ΠΛH =Θ≠38.6 kJ/mol
D.“―÷Σ25ΓφΓΔ101kPaΧθΦΰœ¬ΘΚ4Al(s)+3O2(g)=2Al2O3(s) ΠΛH =Θ≠2834.9 kJ/molΘ§4Al(s)+2O3(g)=2Al2O3(s) ΠΛH =Θ≠3119.1 kJ/molΘ§‘ρO2±»O3Έ»Ε®
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩMgCO3ΚΆCaCO3ΒΡΡήΝΩΙΊœΒ»γΆΦΥυ Ψ(MΘΫCaΓΔMg)ΘΚ
ΓΓΓΓM2+(g)ΘΪCO32-(g)ΓΓ![]() ΓΓM2+(g)ΘΪO2(g)ΘΪCO2(g)
ΓΓM2+(g)ΘΪO2(g)ΘΪCO2(g)
![]() ΓΓΓΓΓΓ
ΓΓΓΓΓΓ![]() ΓΓΓΓ
ΓΓΓΓ![]()
“―÷ΣΘΚάκΉ”ΒγΚ…œύΆ§ ±Θ§ΑκΨΕ‘Ϋ–ΓΘ§άκΉ”Φϋ‘Ϋ«ΩΓΘœ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «
A. ΠΛH1(MgCO3)ΘΨΠΛH1(CaCO3)ΘΨ0
B. ΠΛH2(MgCO3)ΘΫΠΛH2(CaCO3)ΘΨ0
C. ΠΛH1(CaCO3)Θ≠ΠΛH1(MgCO3)ΘΫΠΛH3(CaO)Θ≠ΠΛH3(MgO)
D. Ε‘”ΎMgCO3ΚΆCaCO3Θ§ΠΛH1ΘΪΠΛH2ΘΨΠΛH3
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΗΏ¬·ΝΕΧζΙΐ≥Χ÷–ΖΔ…ζΖ¥”ΠΘΚ ![]() Fe2O3(s)ΘΪCO(g)
Fe2O3(s)ΘΪCO(g)![]()
![]() Fe(s)ΘΪCO2(g)Θ§ΗΟΖ¥”Π‘Ύ≤ΜΆ§Έ¬Ε»œ¬ΒΡΤΫΚβ≥Θ ΐΦϊ”“±μΓΘœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©
Fe(s)ΘΪCO2(g)Θ§ΗΟΖ¥”Π‘Ύ≤ΜΆ§Έ¬Ε»œ¬ΒΡΤΫΚβ≥Θ ΐΦϊ”“±μΓΘœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©
Έ¬Ε»T/Γφ | 1000 | 1150 | 1300 |
ΤΫΚβ≥Θ ΐK | 4.0 | 3.7 | 3.5 |
A. ”…±μ÷– ΐΨίΩ…≈–ΕœΗΟΖ¥”ΠΘΚΖ¥”ΠΈοΒΡΉήΡήΝΩΘΦ…ζ≥…ΈοΒΡΉήΡήΝΩ
B. 1000Γφœ¬Fe2O3”κCOΖ¥”ΠΘ§t min¥οΒΫΤΫΚβ ±c(CO) =2ΓΝ10-3 mol/LΘ§‘ρ”ΟCO2±μ ΨΗΟΖ¥”ΠΒΡΤΫΨυΥΌ¬ ΈΣ2ΓΝ10Θ≠3/t molΓΛLΘ≠1ΓΛminΘ≠1
C. ΈΣΝΥ ΙΗΟΖ¥”ΠΒΡK‘ω¥σΘ§Ω…“‘‘ΎΤδΥϊΧθΦΰ≤Μ±δ ±Θ§‘ω¥σc(CO)
D. ΤδΥϊΧθΦΰ≤Μ±δ ±Θ§‘ωΦ”Fe2O3ΒΡ”ΟΝΩΘ§≤ΜΡή”––ßΫΒΒΆΝΕΧζΈ≤Τχ÷–COΒΡΚ§ΝΩ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com