
2.25g��
| ||
| 8.0 |
| 2.25g |
| 18g/mol |
| 10 |
| 8 |
| 250mL |
| 20mL |
| 10 |
| 8 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������������ϡ���ᷴӦ��Fe+4H++NO3-=Fe3++2H2O+NO�� |
| B��������SO2����ͨ��NaClO��Һ�У�SO2+ClO-+H2O=SO32-+2HClO |
| C��������Һ�м�����������������Һ��Ba2++OH-+H++SO42-=BaSO4+H2O |
| D����Mg��OH��2��Һ�е���FeCl3��Һ��3 Mg��OH��2��s��+2 Fe3+?2 Fe��OH��3��s��+3Mg2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
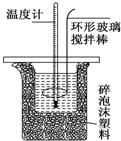 �ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��| �¶� ʵ����� | ��ʼ�¶�T1/�� | ��ֹ�¶�T2/�� | �¶Ȳ�ƽ��ֵ��T2-T1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
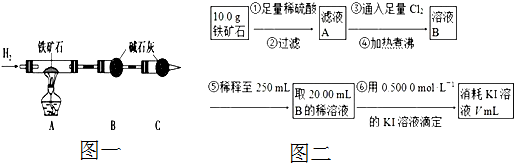
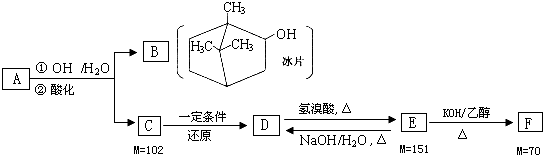
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
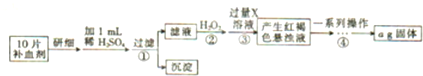
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
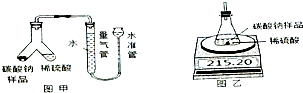 ij���������ð�������Ĵ����к��������Ȼ������ʣ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�ij��ѧ�о���ѧϰС������йط���������ͼʵ�飺
ij���������ð�������Ĵ����к��������Ȼ������ʣ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�ij��ѧ�о���ѧϰС������йط���������ͼʵ�飺| ʱ��/s | 0 | 5 | 10 | 15 |
| ����/g | 215.20 | 211.40 | 208.60 | 208.60 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | ���³�ѹ�£�Y�����ǵ���ɫ���壬���ڻ�ɽ�������� |
| Z | Z��Yͬ���ڣ�Z�ĵ縺�Դ���Y |
| W | W��һ�ֺ��ص�������Ϊ63��������Ϊ34 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ������ | Ԫ�ر�� | |||||||
| A | B | C | D | E | F | G | H | |
| ԭ�Ӱ뾶��nm�� | 0.102 | 0.110 | 0.117 | 0.074 | 0.075 | 0.071 | 0.099 | 0.186 |
| ����ϼ� | +6 | +5 | +4 | +5 | +7 | +1 | ||
| ��ͻ��ϼ� | -2 | -3 | -4 | -2 | -3 | -1 | -1 | 0 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com