
����Ŀ��ij������ȤС����Ҫ�� 18 mol/L ��Ũ��������80mL 3.0 mol/L ϡ�����ʵ�鲽�����£� �� ��������Ũ�������� �� ��ȡһ�������Ũ���� �� ϡ�� �� ��©�� ת�ơ�ϴ�Ӣ� ���ݡ�ҡ�� �ش��������⣺
(1)����Ũ����������__________ mL�� ��ȡŨ�������õ���Ͳ�Ĺ����___________ (�����б�����) ��
A��10 mL B��25 mL C��50 mL D��100 mL
(2)�ڢݲ�ʵ��IJ����Ǽ���������ƿ��ע������ˮ����̶���__________�� ����___________������ƿ�еμ�����ˮ��_________________________Ϊֹ������ƿ������תҡ�Ȳ�ת�����Լ�ƿ�С�
(3)��������������Ƶ�ϡ����Ũ���к�Ӱ�죿 (�� �� ƫ�� ���� ƫС �� �� �� ��Ӱ����)
������ƿ������ˮϴ�Ӻ������������ˮ_________________
����ת������ƿǰ�ձ�����Һδ��ȴ������_________
��ȡ��Ũ�����ϴ����Ͳ������ϴ��Һ�����ձ�___________��
�ܶ��ݽ���ʱ���ӿ̶���_______________
���𰸡�16.7 B 1�� 2 cm ��ͷ�ι� ��Һ�����ʹ���̶������� ��Ӱ�� ƫ�� ƫ�� ƫ��
��������
��1������80mL3.0mol/Lϡ���ᣬ��ѡ��100mL������ƿ�������Ҫ18mol/LŨ��������Ϊ![]() ����Ͳ��ѡ��Ϊ��������ѡ��25mL��Ͳ��
����Ͳ��ѡ��Ϊ��������ѡ��25mL��Ͳ��
��2�����ݲ���Ϊ������������ƿע������ˮ���̶���1-2cm�������ý�ͷ�ι�������ƿ�μ�����Һ����̶������У�
��3����.����ƿ������ˮϴ�Ӻ������������ˮ����Ϊ����ʱ����Ҫ��������ˮ�����Բ�Ӱ�����ƽ����
��.��ת������ƿǰ�ձ�����Һδ��ȴ�����£���������������ԭ������ȴ�������С��Ũ��ƫ��
��.��Ͳ��������ʽ������ȡ��Ũ�����ϴ����Ͳ������ϴ��Һ�����ձ���������ȡŨ��������ƫ�����ս��ƫ��
��.���ݽ���ʱ���ӿ̶��ߣ�������Һ���ƫС��Ũ��ƫ��


 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������
��I2 ��Br�� ��Na�� ��Fe2�� ��H�� ��Fe3��
��Mg ��Cl2 ��HCl ��H2O S SO2
�����ڷ�Ӧ��ֻ������ԭ������ �� ֻ�������������� �� �ȿ����������ֿ�����ԭ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����( )
A.��ˮ�����Ժܲ����ˮ���ǵ����
B.�ж�ij�������Ƿ�Ϊ����ʣ�Ӧ������һ���������ܷ����
C.�ᡢ���������ڵ���ʣ����������ﶼ���ǵ����
D.NaCl��HCl���ǵ���ʣ�������������״̬�¶��ܵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������CoCl2��6H2O��NH4Cl��H2O2��Ũ��ˮΪԭ�ϣ��ڻ���̿���£��ϳɳȻ�ɫ����Co(NH3)6Cl3��ʵ�����̺�װ��ͼ���£�
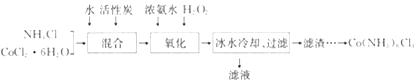
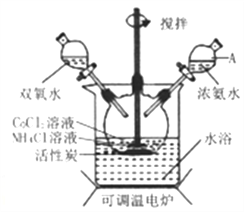
��֪���ٲ�ͬ�¶��£�Co(NH3)6Cl3��ˮ���ܽ��������ͼ��ʾ��
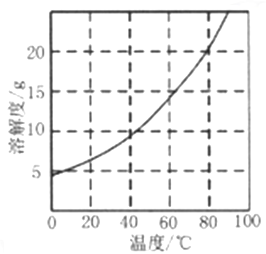
��Co(NH3)6Cl3 ����Һ����Ҫ��Co(NH3)63+��Cl- ��ʽ����,��������Ũ���������ڽᾧ������
��Ksp[Co(OH)2]=1.09��10-15��Ksp[Co(OH)3]=1.6��10-44��
�ش��������⣺
(1)ͼ������A��������_______;��ʵ�������NH4Cl������Ӧ���⣬���ɷ�ֹ�ڵμӰ�ˮʱ���ɷۺ�ɫCo(OH)2����,��ԭ����_______��
(2)����������ͼ��ʾװ���н��У�����Co(NH3)6Cl3��
������ʱ���ȼ��백ˮ,�ټ���H2O��Ŀ����_______;�÷�Ӧ�����ӷ���ʽΪ_______��
�ڷ�Ӧʱ��Ҫ�����¶���50~60��֮��,�¶Ȳ��ܹ��ߵ�ԭ����_______��
(3)��ˮ��ȴ��Ŀ����_______ �������������е���Ҫ����Ϊ_______��
(4)������ɹ��˺�����������ȡCo(NH3)6Cl3��ʵ�鷽���������������ˮ�У���ֽ���,���ȹ��ˣ�_______�����������Ҵ�ϴ�ӣ����¸��
(5)����ʵ�����������ʹ�û���̿������,���õ��Ϻ�ɫ����Co(NH3)5Cl3(M=250.5g��mol-1)��ȡ2.505g�Ϻ�ɫ����,����ˮ���������AgNO3��Һ,���ɰ�ɫ����2.870g,д���÷�Ӧ�Ļ�ѧ����ʽ��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.�л���A�Ľṹ��ʽ�� ��������ͨ����ͬ�ķ�Ӧ�õ��������ʣ�
��������ͨ����ͬ�ķ�Ӧ�õ��������ʣ�
B. 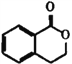 C.
C. 
D.  E.
E. 
��1��A�к��������ŵ����ƣ�_____________________________��
��2��д����A��ȡB�Ļ�ѧ����ʽ��_____________________________��
��3��д��A��ȡC��D���������ʵ��л���Ӧ���ͣ�
A��C��_____________��Ӧ��A �� D ��_________________��Ӧ
��4��д��һ�ּȿ��Կ��������ֿ��Կ������࣬�ҷ����б�������������ȡ������A��ͬ���칹��Ľṹ��ʽ��______________________________��
��.F��G�����л��������ش��������⣺
��1����֪��6.0g������F��ȫȼ������8.8gCO2��3.6gH2O��F������������������ܶ�Ϊ30����F�ķ���ʽΪ__________________________��
��2��G�ķ���ʽ��C8H8Br2����G��ͬ���칹���У�������һ�����IJ���ֻ��һ�ֵĹ���_______�������к˴Ź�������������壬�ҷ���ְ��Ϊl��l����_______����ṹ��ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ɫ��Һ�п��ܺ���Mg2+��Ba2+��Cl-��HCO3-�е�һ�ֻ������ӡ�Ϊȷ����ɷ֣���������ʵ�飺
ʵ��1��ȡ10mL��ɫ��Һ���μ�����ϡ��������������
ʵ��2����ȡ10mL��ɫ��Һ������������Na2SO4��Һ���а�ɫ�������ɡ�
ʵ��3����ʵ��1�����Һ����ƿ�У�����ƿ����μ���NaOH��Һ���μӹ����в������������������NaOH��Һ������Ĺ�ϵ����ͼ��ʾ��

�ش��������⣺
(1)ԭ��Һ�в����ڵ�������__________�����ڵ�������________________��������ڵ������ӵķ�����________________________________________
(2)ʵ��3�У�ͼ����OA�η�Ӧ�����ӷ���ʽΪ____________________________��
(3)����ͼ�����ԭ��Һ��Mg2+�����ʵ���Ũ��_______��(д���������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������е�����������( )
A.����״̬��KOH
B.ʯī��
C.��̬KCl
D.ϡH2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����·�Ӧ�ᵼ����Һ�����Լ��������Ե���( )
A.Na2SO4��Һ����BaCl2����
B.Ba(OH)2��Һ�м���CuSO4(����)����
C.NaOH��Һͨ��HCl
D.H2O���NaCl����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�ڱ�״���£���CO��CO2��ɵĻ������8.96L��������16g���˻������CO��CO2�����ʵ���֮����_________��CO�����������_________����������ƽ��Ħ��������____________��
(2) 1.15g�����Ƹ�ˮ��Ӧ���õ�100mL��Һ���Լ��㣺
�����ɵ������ڱ�״������_________________
�ڷ�Ӧ��������Һ�����ʵ���Ũ����______________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com