
����Ŀ��������һ�������Դ,��������ȡ�봢��������Դ����������о��ȵ㡣
��1���Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ������֪:
CH4(g)+H2O(g) = CO(g)+3H2(g)��H=+206.2 kJ/mol
CH4(g)+CO2(g) = 2CO(g)+2H2(g)��H=+247.4 kJ/mol
CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ________________________��
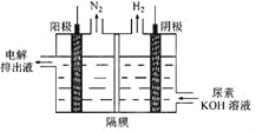
��2���������[CO(NH2)2]�ļ�����Һ�����װ��ʾ��ͼ��ͼ(�����и�Ĥ����ֹ����ͨ��,����������Ϊ���Ե缫)�����ʱ,�����ĵ缫��ӦʽΪ________________________��
��3�� Mg2Cu��һ�ִ���Ͻ�350��ʱ,Mg2Cu��H2��Ӧ,����MgCu2�ͽ���һ�ֽ���Ԫ�ص��⻯��(���������������Ϊ0.077)��Mg2Cu��H2��Ӧ�Ļ�ѧ����ʽΪ_______________��
���𰸡�CH4(g)+2H2O(g) = CO2(g) +4H2(g)��H=+165.0 kJ/mol CO(NH2)2+8OH--6e- = CO32-+N2��+6H2O 2Mg2Cu+3H2![]() MgCu2+3MgH2
MgCu2+3MgH2
��������
��1�����ø�˹���ɽ�����Ӵ���Ӧ����������Ӧ���������������Ӧ�е�λ����ͨ����Ӽ��ɵ���
��2���������ų�Һ�к��д�����̼���γɷݣ����������������뷴Ӧ��NԪ�صĻ��ϼ����ߣ��Դ�����д�缫��Ӧ�������ܷ�Ӧ������������KOH�Ĺ�ϵ�����
��3��������⻯��ΪRHx������R�����ԭ������Ϊa����![]() =0.077����923x=77a��XΪ�����Ļ��ϼۣ����ۿɵ�x=2��a=24���ʸý����⻯��ΪMgH2��
=0.077����923x=77a��XΪ�����Ļ��ϼۣ����ۿɵ�x=2��a=24���ʸý����⻯��ΪMgH2��
��1����֪��CH4(g)+H2O(g) = CO(g)+3H2(g)��H=+206.2 kJ/mol
��CH4(g)+CO2(g) = 2CO(g)+2H2(g)��H=+247.4 kJ/mol
�ݸ�˹��������![]() 2-������CH4(g)+2H2O(g) = CO2(g) +4H2(g)��H=+165.0 kJ/mol��
2-������CH4(g)+2H2O(g) = CO2(g) +4H2(g)��H=+165.0 kJ/mol��
��ˣ�������ȷ������CH4(g)+2H2O(g) = CO2(g) +4H2(g)��H=+165.0 kJ/mol��
��2���������ų�Һ�к��д�����̼���γɷݣ��������������뷴Ӧ����������ӦʽΪCO(NH2)2+8OH--6e- = CO32-+N2��+6H2O��
��ˣ�������ȷ������CO(NH2)2+8OH--6e- = CO32-+N2��+6H2O��
��3��������⻯��ΪRHx������R�����ԭ������Ϊa����![]() =0.077����923x=77a��XΪ�����Ļ��ϼۣ����ۿɵ�x=2��a=24���ʸý����⻯��ΪMgH2���ʷ�Ӧ����ʽΪ2Mg2Cu+3H2
=0.077����923x=77a��XΪ�����Ļ��ϼۣ����ۿɵ�x=2��a=24���ʸý����⻯��ΪMgH2���ʷ�Ӧ����ʽΪ2Mg2Cu+3H2![]() MgCu2+3MgH2��
MgCu2+3MgH2��
��ˣ�������ȷ������2Mg2Cu+3H2![]() MgCu2+3MgH2��
MgCu2+3MgH2��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����12�֣��±�ΪԪ�����ڱ���һ���֣������Ԫ���������ڱ��е�λ�ã��û�ѧ����ش��������⣺

��1��������������ԭ�Ӱ뾶�ɴ�С��˳��Ϊ___________________��
��2����������������ۺ������������ǿ������˳����____________��
��3�����������������е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________��
��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________��
a.MnO2 b.FeCl3c.Na2SO3d.KMnO4
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ_____________________��
N�����ĵ��ʵĻ�ѧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ī������������������Һ�ⶨ±���Ӻ����ij����ζ���������ˮFeCl3���ȱ���Ϸ�����Ӧ:2FeCl3 +C6H5Cl ![]() 2FeCl3+C6H4C12+HC1���������ɵ�HC1��ˮ���գ�����Ī���������ˮFeCl3��ת������ͬʱ�õ����õĻ�ԭ��FeCl2��������ͼװ������������ƿ�з���162.5g��ˮFeCl3��225g�ȱ������Ʒ�Ӧ�¶���130���¼���3h����ȴ�����ˡ�ϴ�ӡ�����õ��ֲ�Ʒ���й�����������
2FeCl3+C6H4C12+HC1���������ɵ�HC1��ˮ���գ�����Ī���������ˮFeCl3��ת������ͬʱ�õ����õĻ�ԭ��FeCl2��������ͼװ������������ƿ�з���162.5g��ˮFeCl3��225g�ȱ������Ʒ�Ӧ�¶���130���¼���3h����ȴ�����ˡ�ϴ�ӡ�����õ��ֲ�Ʒ���й�����������
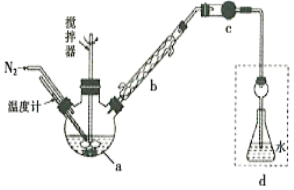

�ش���������:
��1������a��������________����������������_______��
��2����Ӧ����������Ҫ����ͨ��N2��Ŀ����__________������cʢװ���Լ���________(�����)��
A����ʯ�� B��Ũ���� C����ˮ�Ȼ��� D���轺
��3����δ���Һ�л��չ������ȱ���_____________
��4�����и�װ��(ʢ������ˮ)�ܴ���ͼ�����߿��ڲ��ֵ���_________(�����)��
��5������ƿ�ڵ���Һϡ����1000mL������ȡ10. 00mL����0. 2000mol��L-1AgNO3��Һ���еζ�����K2CrO4��Һ��ָʾ�����յ�����Ϊ______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������۷�������ͭ���������Ļ��Һ�У���Ӧֹͣ����������������˺�ȡ������Һ�μ�ϡ���ᣬ������������������һ������(�� )
A.����ͭ
B.����ͭ����
C.ͭ
D.��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��D���ɳ����Ķ����ڷǽ���Ԫ���γɵĵ��ʣ�������A�ǵ���ɫ��ĩ��B��D�����壬F��G��H����ɫ��Ӧ��Ϊ��ɫ��ˮ��Һ���Լ��ԣ�E��Ư���ԡ�����֮���ת����ϵ��ͼ��ʾ�����ֲ��P��Ӧ��������ȥ�����ش��������⣺
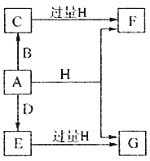
��1��A����Ԫ�������ڱ��е�λ��Ϊ______________��C�ĵ���ʽΪ_______________��
��2��A��H�ڼ��������·�Ӧ�Ļ�ѧ����ʽΪ__________________��
��3����A���ڷ��ڵ�G��Һ�п����Ƶû�����I��I��������Һ�в��ȶ��������ɵ����ʵ�����A��E��I����������������A��E�����ӷ���ʽΪ____________________��I����ǿ��ԭ�����ڷ�֯����ֽ��ҵ����Ϊ���ȼ�����I��Һ��ͨ�������ɷ�����Ӧ���μӷ�Ӧ��I�����������ʵ�����Ϊ1:4���÷�Ӧ�����ӷ���ʽΪ__________________��
��4������0.4molF��0.1molG�Ļ����Һ�м���������ᣬ��ȫ��Ӧ���ռ���aL����C����״������ȡ��Ӧ�������Һ���������FeCl3��Һ���õ�����3.2g����a=____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1L���ݵ��ܱ������У�����֤�����淴ӦN2+3H2![]() 2NH3�Ѵﵽƽ��״̬����
2NH3�Ѵﵽƽ��״̬����
A. c��N2����c��H2����c��NH3��=1��3��2
B. һ��N��N ���ѵ�ͬʱ����3��H-H����
C. ������������ʱ�����������ܶȲ��ٸı�
D. v����N2���T2 v�棨NH3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��д��ȷ���ǣ� ��
A. �ô����ȥˮ����CaCO3+2H+=Ca2++H2O+CO2��
B. ��ͭΪ�缫��ⱥ��ʳ��ˮ��2Cl-+2H2O![]() Cl2��+H2��+2OH-
Cl2��+H2��+2OH-
C. FeBr2��Һ��ͨ�����Cl2��2Fe2++Cl2=2Fe3++2Cl-
D. ��FeCl3��Һ��ʴӡˢ��·����ͭ�䣺2Fe3++Cu=2Fe2++Cu2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ����Ҫ�Ļ���ԭ�ϣ�����ϩΪԭ���ڲ�ͬ�����¿ɺϳ��������ʣ���������δ�������

������Ҫ��д����
��1������ϩ�Ľṹ��ʽ��_____________����ȩ�Ľṹ��ʽ��_____________��
��2����Ӧ�ٵĻ�ѧ����ʽ��________________________________����Ӧ������___________��
��3����Ӧ�۵Ļ�ѧ����ʽ��________________________________����Ӧ������___________��
��4����Ӧ�ݵĻ�ѧ����ʽ��________________________________����Ӧ������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W����������һ�������¾�����ͼ��ʾ��ת����ϵ�������ж���ȷ����
![]()
A. ��ͼ�з�Ӧ��Ϊ��������ԭ��Ӧ����WΪһԪǿ��ʱ����X������NaAlO2
B. ��ͼ�з�Ӧ��Ϊ��������ԭ��Ӧ����WΪһԪǿ��ʱ����X������NH3
C. ��ͼ�з�Ӧ��Ϊ������ԭ��Ӧ����WΪ�ǽ�������ʱ����Z������CO2
D. ��ͼ�з�Ӧ��Ϊ������ԭ��Ӧ����WΪ��������ʱ����Z������FeCl3
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com