
����Ŀ��298Kʱ����20mLcmol��L��1KOH��Һ�еμ�0.1mol��L��1HCOOH��Һ�������Һ��ˮ���������������Ũ����μӼ���(����)��Һ���(V)�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
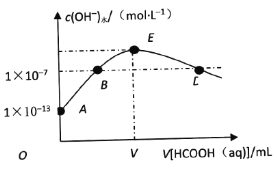
A. ����KOH��Һ��Ũ��c��0.01mol��L��1
B. B���Ӧ����Һ�У�c(K��)��c(HCOO��)
C. E���Ӧ����Һ�У�c(OH��)��c(H��)��c(HCOOH)
D. ��D���Ӧ�ļ�����Һ���ΪV1mL����HCOOH����ƽ�ⳣ��![]()
���𰸡�D
��������
�۲�ͼ���֪��E���ʾKOH��HCOOHǡ����ȫ��Ӧ����HCOOK����ʱ��Һ�ʼ��ԣ�B���Ӧ��Һ�е�������KOH��HCOOK����Һ�Լ��ԣ�D���Ӧ��Һ�е�������HCOOH��HCOOK�������ԣ��ݴ˷������
A������ͼ��ʼʱ��A���pH=13��˵��KOH��Һ��Ũ��c��0.1mol��L��1����A����
B��B���Ӧ��Һ�е�������KOH��HCOOK����Һ�Լ��ԣ�c(OH��)��c(H��)�����ݵ���غ��У�c(K��)��c(HCOO��)����B����
C��E��ΪKOH��HCOOHǡ����ȫ��Ӧ����HCOOK����ʱ��Һ�ʼ��Ը��������غ���c(OH��)=c(H��)+c(HCOOH)����c(H��)��c(HCOOH)��һ����ȣ���C����
D��D���Ӧ��Һ�е�������HCOOH��HCOOK�������ԣ�����c(K��)= c(HCOO��)=![]() =
= ![]() mol��L��1��c(HCOOH)=
mol��L��1��c(HCOOH)=![]() =
= ![]() mol��L��1����HCOOH����ƽ�ⳣ��
mol��L��1����HCOOH����ƽ�ⳣ��![]() =
=![]() =
= ![]() ����D��ȷ��
����D��ȷ��
��ѡD��


 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��FeSO4��7H2O��ҽѧ�ϳ�������Ѫ����ij����С��ͨ����KMnO4�ζ�����õ�FeSO4��Һ���ⶨij��Ѫ����FeSO4�ĺ�����ʵ����������0.01 mol/L 1000 mL��KMnO4��Һ��
(1)��Ҫ�IJ����������ձ���_______ ��_______ ��_______��
(2)��������ƽ��ȡKMnO4���������Ϊ_______g��
(3)���в���ʹʵ����ƫ����� _______��
A.����ƿδ���� B.δϴ���ձ��Ͳ�����
C.����ʱ��������ƿ�̶��� D.���ձ��ܽ�ʱ��������Һ�彦��
(4)�ֲⶨ��Ѫ����FeSO4�ĺ�����ʵ�鲽�����£�
a.ȡ8Ƭ��Ѫ����Ʒ��ȥ���¡���ĥ���ܽ⡢���ˣ�����Һ���Ƴ�250 mL��Һ
b.ȡ������Һ25 mL����ƿ�У��������������ữ���μ�0.01 mol/L��KMnO4��Һ����ӦΪ10 FeSO4+2KMnO4+8H2SO4=5Fe2(SO4)3 +2MnSO4+K2SO4+8H2O����¼�ζ��յ�ʱ����KMnO4��Һ��������ٶ�ҩƷ�������ɷֲ���KMnO4��Ӧ����
c.�ظ�����b 2-3�Σ�ƽ������KMnO4��Һ20.00 mL��
�ò�Ѫ����FeSO4�ĺ���Ϊ _______ mg/Ƭ��д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ϡ��Ԫ����Ԫ�����ڱ��е�IIIB���֡��ƺ���ϵԪ�ص��ܳơ���������������ϡ���������NdFeB������������ۺϴ����ܣ����㷺Ӧ���ڼ������ͨ����Ϣ�ȸ��¼�����ҵ���ش��������⣺
��1����̬Feԭ�ӵļ۵����Ų�ʽΪ____�������ܡ���Ԫ�����ʷdz����ƣ�ԭ�Ӱ뾶�ӽ������μ�С��NiO��FeO�ľ���ṹ�������Ȼ�����ͬ������NiO____���>����<����=����FeO��
��2�������黯���![]() ����һ�����ͻ�ѧ������ϣ���û�������ӻ�Ϊ�ȵ�������л���Ϊ___���ѧʽ���������������N��Bԭ�ӵ��ӻ���ʽ�ֱ�Ϊ___��___��
����һ�����ͻ�ѧ������ϣ���û�������ӻ�Ϊ�ȵ�������л���Ϊ___���ѧʽ���������������N��Bԭ�ӵ��ӻ���ʽ�ֱ�Ϊ___��___��
��3��![]() �׳�Ħ���Σ������
�׳�Ħ���Σ������![]() ���ԣ�Ħ���β���ʧˮ�����ױ������������ڻ�ѧ����ʵ���г���������Fe��II���ı���Һ���Է�����������茶��������������ȶ����ڵ�ԭ��______
���ԣ�Ħ���β���ʧˮ�����ױ������������ڻ�ѧ����ʵ���г���������Fe��II���ı���Һ���Է�����������茶��������������ȶ����ڵ�ԭ��______
��4����������õ�ϡ������֮һ������Ϊ������ϵ����ԭ�����������ܶѻ���ʽ���ӡ�����������![]() ��ÿ����������___����ԭ�ӣ��谢���ӵ�����Ϊ
��ÿ����������___����ԭ�ӣ��谢���ӵ�����Ϊ![]() ��������ϵ��ܶ�Ϊ___
��������ϵ��ܶ�Ϊ___![]() ��Nd�����ԭ������ΪM���г��������ʽ��
��Nd�����ԭ������ΪM���г��������ʽ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������Һ��ͨ����������������ͬʱ�õ����ᡣԭ����ͼ��ʾ��ͼ��HA��ʾ������ӣ�A-��ʾ��������ӣ������Ħ������Ϊ90g/mol�������й�˵������ȷ����( )

A. �缫aΪ�������ų�H2
B. ͨ��һ��ʱ���������Һ��pH����
C. A-ͨ�������ӽ���Ĥ����������Ũ����
D. ��400mLl0g/L������Һͨ������·ͨ��0.5mole-ʱŨ�ȣ�����Ϊ145g.L-1����Һ����仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ۺ�����CO2��CO�Թ�����̼�������Ҫ���塣
��1��H2 ��CO�ϳɼ״���ӦΪ��CO��g��+2H2��g��![]() CH3OH��g�� ��H��0���ں��£����Ϊ2L���ܱ������зֱ����1.2mol CO��1mol H2��10min��ﵽƽ�⣬��ú���0.4mol CH3OH��g������ﵽƽ��ʱCO��Ũ��Ϊ_______��10min����H2��ʾ�Ļ�ѧ��Ӧ����Ϊ_______����Ҫ�ӿ�CH3OH���������ʲ����CO��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_______����һ�ֺ����Ĵ�ʩ����
CH3OH��g�� ��H��0���ں��£����Ϊ2L���ܱ������зֱ����1.2mol CO��1mol H2��10min��ﵽƽ�⣬��ú���0.4mol CH3OH��g������ﵽƽ��ʱCO��Ũ��Ϊ_______��10min����H2��ʾ�Ļ�ѧ��Ӧ����Ϊ_______����Ҫ�ӿ�CH3OH���������ʲ����CO��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_______����һ�ֺ����Ĵ�ʩ����
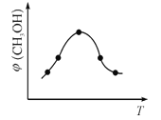
��2��������̼�ϳɼ״���̼���ŵ��·���CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ��CO2(g) +3H2(g) ![]() CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK=________��
���ں����ܱ�������ʹCO2��H2�����ʵ���֮��Ϊ1��3��,����������Ӧ����Ӧ�����в�ü״������������(CH3OH)�뷴Ӧ�¶�T�Ĺ�ϵ����ͼ��ʾ���� ��H _________0����������������С������
��3�� ����ͼ��ʾ������ȱ��������[��Fe0.9O]��ʵ��CO2���ۺ����á���˵����ת����2���ŵ�_____________������1 molȱ��������[Fe0.9O]������CO2��ȫ��Ӧ������________mol C(̼)��
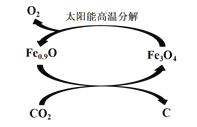
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ��Ӧ�����ӷ���ʽ��ȷ���� �� ��
A.��С�մ�����θ����ࣺHCO3-+H+=CO2��+H2O
B.��̼��þ�еμ�ϡ���CO32-+2H+=CO2��+H2O
C.����ˮ�еμ��Ȼ�����Al3++4OH-=[Al(OH)]4-
D.����������Һ��ϡ���ᷴӦ��Ba2++SO42-+H++OH-=BaSO4��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʱ��2017��10��4�գ�������һ��С����ײ���¼���ײ���ص�Ϊ�ҹ�������������س�����40���ﴦ����ը�����൱��540t TNT���ܿ�����δȼ������ʯ�䵽���档ȫ�������ռ���4������ʯ��Ʒ�����Ǵ��¿ɷ�Ϊ�����ࣺʯ��ʯ(��Ҫ�ɷ��ǹ�����)������ʯ(�����Ͻ�)����ʯ����ʯ(�������λ����)��

�ش��������⣺
(1)��̬��ԭ�Ӽĵ����Ų�ʽΪ[Ar]_______��
(2)TNT�Ľṹ��ʽ��ͼ��ʾ��

��TNT������̼ԭ���ӻ�������____��
��TNT���۵�����������۵�__(����������������)��������___��
(3)ʯ��ʯ�й�����֮һ��Ca2SiO4��
SiO44�������幹����_____���縺�ԣ�Si___(����������������������)O��
(4)K3[Fe(CN)6](���軯��)��Һ���Լ�������ʯ����Ԫ�ؼ�̬��
�����軯���в����ڵ���������__(����ĸ)��
a.���Ӽ� b.���Լ� c.�Ǽ��Լ� d.���� e.��λ�� f.���
��1mol [Fe(CN)6]3��������ĿΪ_____��
��CN��������������γ�����̼ԭ���ṩ�µ��Ӷԣ������ǵ�ԭ�ӣ���ԭ����___��
(5)��������ɴŲ���M���侧����ͼ��ʾ���þ����е�����ԭ�Ӹ���֮��Ϊ____��

(6)������Ķѻ���ʽΪ�����������ܶѻ�����֪�������ܶ�Ϊ�� g��cm��3��NA���������ӵ�������ֵ���ú�����NA�Ĵ���ʽ��ʾ���������������������ԭ��֮��ĺ˼��D��__nm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ṥҵ��ϳɰ���ҵ������أ����������ǹ�ҵ��������ȡ�������Ҫ;��������Ҫ������ͼ��
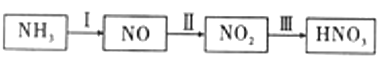
I. ��һ�ݻ�Ϊ2L���ܱ������ڼ���2molN2��6molH2����һ�������ºϳɰ�������֪N2(g)+3H2(g)![]() 2NH3 ��H<0����Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ
2NH3 ��H<0����Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ

��1������ͼʾ������ӷ�Ӧ��ʼ����4���Ӵﵽƽ��ʱ��ƽ����Ӧ������(N2)Ϊ______
��2���ﵽƽ���5����ĩ�������������������䣬ֻ�ı䷴Ӧ�¶ȣ���NH3�����ʵ���Ũ�Ȳ�����Ϊ________(�����)��
a. 0. 8mol/L b.1.0mol/L. c.1.2mol/L d. 2. mol/L
��3���ﵽƽ���5����ĩ�������������������䣬ֻ�������������С����ƽ��ʱNH3��Ũ��ǡ��Ϊԭ����2�����������_________(ѡ����������������������С����)����֮һ����ԭ�����
��.ijС��ͬѧ��̽����NH3��ȡHNO3�Ĺ��̣���ư���ͼ��ʾװ�ý������顣
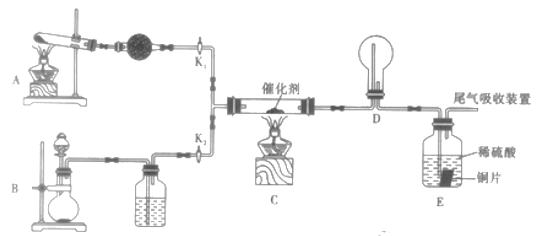
��1��Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________��
��2��Bװ���Ʊ�������Ϊ_________________��(�����)
A . NH3 B Cl2 C.O2 D. HCl
��3����ʵ�����ܹ�֤���ж�����������������Ϊ_______________________��
��4����֪E��װ��2mol/L������150mL������ͭƬ����E�г�����Һ����ɫ���������з�Ӧ��ͭ��ת��ΪCuSO4����������Ҫ���������_______L����(���������Ӧ����ȫ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
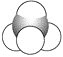
����Ŀ���л���ı�ʾ�������ֶ����������dz����л���ı�ʾ������
��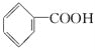 ����
����![]() ����CH4
����CH4
��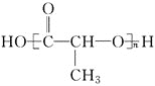 ����
����
��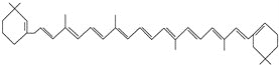
��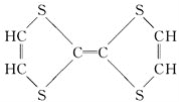
�� ����
����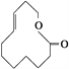
��
(1)������ʾ���������ڽṹ��ʽ��Ϊ__________��
���ڽṹʽ��Ϊ________��
���ڼ���ʽ��Ϊ________��
���ڱ���ģ�͵�Ϊ________��
�������ģ�͵�Ϊ________��
(2)д����ķ���ʽ��________��
(3)д�����й����ŵĵ���ʽ��________��________��
(4)�ڵķ���ʽΪ________�����ʽΪ________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com