
����Ŀ��25��ʱ���ֱ�������pH��Ϊa��CH3COOH��Һ��HCN��Һ�м�ˮϡ�ͣ�ϡ��������Һ��pH�仯����Һ����Ĺ�ϵ��ͼ��ʾ��
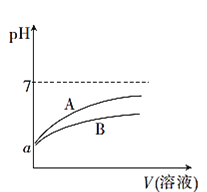
��֪25��ʱ��HCN�ĵ���ƽ�ⳣ��Ka=6.2��10-10��CH3COOH�ĵ���ƽ�ⳣ��Ka=1.7��10-5
(1)��ʾCH3COOH��Һ��pH�仯���Ƶ�������____(����A������B��)��
(2)pH��Ϊa��CH3COOH��Һ��HCN��Һ�����ʵ����ʵ���Ũ�Ƚϴ����____(�ѧʽ)��
(3)25��ʱ����Ũ�ȵ�NaCN��Һ��pH___����>��=������<��)CH3 COONa��Һ��pH��
(4)25��ʱ����20mL0.01mol��L-1CH3COOH��Һ����μ���0.01mol��L-1KOH��Һ����pH=7ʱ������KOH��Һ�����____(�����<��)20mL��
(5)����ͬŨ�ȵ�AgNO3��Һ�ֱ�ζ�Ũ����ͬ�ĺ�Cl����Br����I���Ļ����Һ����ͼ��ȷ�����ȳ�����������______��(�����ӷ���)
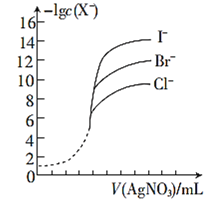
���𰸡�A HCN > < I-
��������
��֪25��ʱ��HCN�ĵ���ƽ�ⳣ��Ka=6.2��10-10��CH3COOH�ĵ���ƽ�ⳣ��Ka=1.7��10-5��HCN�ĵ���ƽ�ⳣС��CH3COOH�ĵ���ƽ�ⳣ������CH3COOH�����Ա�HCN������ǿ������Խǿ�����Ӧ��������ӵ�ˮ������Խ���������ܽ�ƽ���Ӧ�á�
��1��CH3COOH�����Ա�HCN������ǿ��pH���ʱ����ˮϡ����ͬ�ı��������Խ�ǿ��pH�仯��A��ʾCH3COOH��Һ��pH�仯���Ƶ����ߣ�
��2������HCN�ĵ���ƽ�ⳣС��CH3COOH�ĵ���ƽ�ⳣ���������ʵ���Ũ����ȵ�CH3COOH��Һ��HCN��Һ��CH3COOH����Һ�е������c(H+)������HCN����Һ�е������c(H+)����pH��ȵ�CH3COOH��Һ��HCN��Һ�����ʵ����ʵ���Ũ�Ƚϴ����HCN��Һ��
��3������Խǿ�����Ӧ��������ӵ�ˮ������Խ��������CH3COOH�����Ա�HCN������ǿ������CN-��ˮ��������CH3COO-��ˮ��ˮ��ǿ����25��ʱ����Ũ�ȵ�NaCN��Һ��pH>CH3COONa��Һ��pH��
��4��25��ʱ����20mL0.01mol��L-1CH3COOH��Һ����μ���0.01mol��L-1KOH��Һ��������KOH��Һ�����Ϊ20mLʱ��CH3COOH��KOHǡ����ȫ��Ӧ����CH3COOK��CH3COOK��ǿ�������Σ���Һ�ʼ��ԣ��ʵ�pH=7ʱ������KOH��Һ�����<20mL��
��5����ͼ���֪-lgc(X-)��ֵԽ����c(X-)ԽС����c(Ag+)��ͬʱ����ʼ��������c(I-)��С��������ͬŨ�ȵ�AgNO3��Һ�ֱ�ζ�Ũ����ͬ�ĺ�Cl����Br����I���Ļ����Һ�����ȳ�����������I- ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij��ȼ�Ϻ���̼���⡢������Ԫ�ء�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������O2��ȼ�գ���ʹ����������ȫ��ͨ����ͼ��ʾװ���У��õ���������е�ʵ����(���������������ȫ������)��
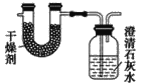
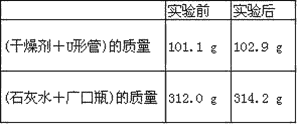
����ʵ��������
(1)ʵ����Ϻ���������ˮ������Ϊ________g��������ƿ������һ�����Σ�������Ϊ_____________g��
(2)���ɵ�ˮ����Ԫ�ص�����Ϊ_______g��
(3)���ɵĶ�����̼��̼Ԫ�ص�����Ϊ_______ g��
(4)��ȼ����̼Ԫ������Ԫ�ص�������Ϊ_______��
(5)��֪����ȼ�ϵ�ÿ�������к���һ����ԭ�ӣ����ȼ�ϵķ���ʽΪ_______���ṹ��ʽΪ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о�С������ú��ȵ��л���A�ϳ���֯�����̿���������M��·������(���ַ�Ӧ�Լ�������δע��)��

��֪����E�ķ���ʽΪC5H8O4���ܷ���ˮ�ⷴӦ���˴Ź���������ʾE��������2�ֲ�ͬ��ѧ��������ԭ�ӣ��������Ϊ3��1��

(R��R�䡢R�������ͬ����ͬ������)��
(1)A�����еĺ��������ŵ�������________________��
(2)D��E��Ӧ�Ļ�ѧ����ʽ��____________��
(3)A��B��Ӧ������Լ���________________��
(4)G��H��Ӧ�Ļ�ѧ����ʽ��____________��
(5)��֪1 mol E��2 mol J��Ӧ����1 mol M����M�Ľṹ��ʽ��________��
(6)E��ͬ���칹�����������ʣ�������NaHCO3��Ӧ����CO2�����ܷ���ˮ�ⷴӦ����ˮ�����֮һ�ܷ���������Ӧ�����ͬ���칹�干��________�֣���������1�ֵĽṹ��ʽ��________��
(7)J�ɺϳɸ߷��ӻ�����ø߷��ӻ�����Ľṹ��ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ɫ���ӷ���Һ�壬�ڻ���������;�㷺��ʵ����ģ��һ�ֹ�ҵ�Ʊ����������Ҫ�������£�
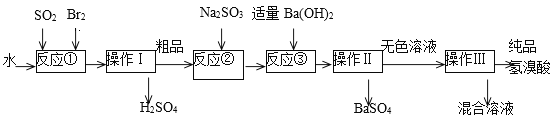
��1��д����Ӧ�ٵ����ӷ���ʽ___________�÷�Ӧ��Ҫ�ڱ�ˮԡ�н��У����ܵ�ԭ����____________��
��2������I������__________��
��3����Ӧ����Na2SO3��Ŀ����_____________________��
��4���������õ��IJ����������ձ���_____________________��
��5����ҵ�������Ƶõ���������е����Ļ�ɫ�����Ǽ�����ͬѧ�����ʵ�����̽����
�ټ�ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3����������֤���ü������õ��Լ�Ϊ_________������������ɹ۲쵽������Ϊ____________________��
����ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ____��������֤���ü�������ķ���Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(SiHCl3)���Ʊ����顢�ྦྷ�����Ҫԭ�ϣ����ڷ�Ӧ2SiHCl3(g)![]() SiH2Cl2(g) ��SiCl4(g)��Ӧ����v��v����v����k��x2SiHCl3��k��xSiH2Cl2xSiCl4��k����k���ֱ�Ϊ��������Ӧ���ʳ�����xΪ���ʵ������������ô�������������ӽ�����֬��������323K��343KʱSiHCl3��ת������ʱ��仯�Ľ����ͼ��ʾ������˵��������ǣ� ��
SiH2Cl2(g) ��SiCl4(g)��Ӧ����v��v����v����k��x2SiHCl3��k��xSiH2Cl2xSiCl4��k����k���ֱ�Ϊ��������Ӧ���ʳ�����xΪ���ʵ������������ô�������������ӽ�����֬��������323K��343KʱSiHCl3��ת������ʱ��仯�Ľ����ͼ��ʾ������˵��������ǣ� ��
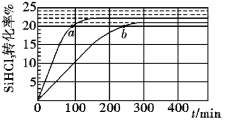
A.�÷�Ӧ������Ӧ��ܴ����淴Ӧ���
B.a��b����Ӧ���ʴ�С��va����vb
C.�¶�һ��ʱʹ�ø����ʴ�������ʹk����k������k����k���ı�ֵ����
D.343Kʱ��Ӧ��ƽ�ⳣ��K=![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��7.8�˹������ƹ����ڳ��µ�ѹ�£���һ������ˮ������Ӧ�����յõ�����16.8�ˣ��˹���������һ������
A.NaOH��H2OB.NaOHC.Na2O2��2H2OD.Na2O2��8H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ������������ϵ��ǣ�������

A.ͼ1��ʾ��ͬ�¶��£���pH��10������������Һ�Ͱ�ˮ�зֱ��ˮϡ��ʱpH�仯���ߣ�����a��ʾ��ˮϡ��ʱpH�ı仯����
B.ͼ2��ʾ�Ѵ�ƽ���ij��Ӧ����t0ʱ�ı�ijһ������Ӧ������ʱ��仯����ı������һ���Ǽ������
C.ͼ3��ʾ��ҵ����CO�����״��ķ�ӦCO(g)��2H2(g)![]() CH3OH(g)���÷�Ӧ����H����91 kJ��mol��1
CH3OH(g)���÷�Ӧ����H����91 kJ��mol��1
D.ͼ4��ʾ10 mL 0.01 mol��L��1����KMnO4��Һ�������0.1 mol��L��1H2C2O4��Һ���ʱ��n(Mn2��)��ʱ��ı仯(Mn2���Ը÷�Ӧ�д�����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25 ��ʱ�������й���Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
A.0.1 mol��L��1(NH4)2Fe(SO4)2��Һ�У�c(![]() )>c(
)>c(![]() )>c(Fe2��)>c(H��)
)>c(Fe2��)>c(H��)
B.pH��11�İ�ˮ��pH��3��������Һ�������ϣ�������Һ�У�c(Cl��)>c(![]() )>c(OH��)>c(H��)
)>c(OH��)>c(H��)
C.��0.1 mol��L��1 Na2CO3��Һ�У�2c(Na��)��c(![]() )��c(
)��c(![]() )��c(H2CO3)
)��c(H2CO3)
D.0.1 mol��L��1�Ĵ�������Һ20 mL��0.1 mol��L��1����10 mL��Ϻ���Һ�����ԣ�c(CH3COO��)> c(Cl��)> c(CH3COOH)> c(H��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б�ʾ��ѧ��Ӧ�����ӷ���ʽ��ȷ����
A.![]() ��Һ�е���ϡ���SO32-+2H+=H2O+SO2��
��Һ�е���ϡ���SO32-+2H+=H2O+SO2��
B.��FeCl3��Һ��ʴͭ��·�壺Cu+2Fe3+=Cu2++2Fe2+
C.̼�������ϡ���CaCO3+2H+=Ca2++H2O+CO2��
D.��������ͭˮ��Һ��Ӧ��2Na+Cu2+=Cu+2Na+
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com