
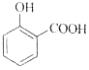
����Ŀ�����л����
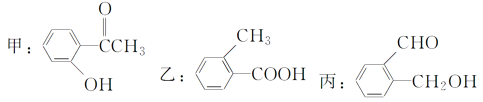
(1)��д�����к��������ŵ����ƣ�___________��__________��
(2)���б�������Щ�����ﻥΪͬ���칹�壺____��______��_____��(�����ף��ң�������ʾ)
(3)��ֱ�д������ס��ҡ������ֻ�����ķ���(ָ����ѡ�Լ�����Ҫ����)��
����ķ������Լ�________________����________________________��
�����ҵķ������Լ�________________����________________________��
������ķ������Լ�________________����________________________��
(4)�밴������ǿ������˳�����мס��ҡ�����˳��_________��
���𰸡�ȩ���� �ǻ� �� �� �� FeCl3��Һ ��FeCl3��Һ��������ɫ��Ϊ�� Na2CO3��Һ ��Na2CO3��Һ�������������ɵ�Ϊ�� ������Һ ��������Һ���ȷ���������Ӧ���DZ� ��>��>��
��������
(1)���ݱ��Ľṹ��ʽ���ж��京�������ŵ����ƣ�
(2)����ͬ���칹���Ƿ���ʽ��ͬ���ṹ��ͬ�Ļ������д���������ʵķ���ʽ��Ȼ�������ʵĽṹ�����жϣ�
(3)���з��ǻ�����FeCl3��Һ����ɫ���Һ����Ȼ�����̼���Ʒ������ֽⷴӦ����CO2���壻���к���ȩ����������������Һ������������ͭ����Һ��Ӧ��
(4)��������Ա�̼��ǿ����ʹʯ����Һ��Ϊ��ɫ�������׳�ʯ̿�ᣬ�������ԣ�����ʹʯ����Һ��ɫ����Ϊ�������ʡ�
(1)���ṹ��ʽΪ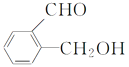 ��������ṹ��ʽ��֪���к�������������Ϊȩ�����ǻ���
��������ṹ��ʽ��֪���к�������������Ϊȩ�����ǻ���
(2)�������ʵĽṹ��ʽ��֪���������ʷ���ʽ����C8H8O2���ṹ��ͬ����˼ס��ҡ�������Ϊͬ���칹�壻
(3)ֻ�м��з��ǻ����������������зֱ����FeCl3��Һ�����л�����FeCl3��Һ����ɫ��˵�����з��ǻ����������� ��ֻ�������ʺ����Ȼ��������������м���Na2CO3��Һ������Na2CO3��Һ���������ݲ�����֤�����к����Ȼ�����������
��ֻ�������ʺ����Ȼ��������������м���Na2CO3��Һ������Na2CO3��Һ���������ݲ�����֤�����к����Ȼ����������� �����к���ȩ���������������м�������������Һ��ˮԡ���ȣ���������������֤������ȩ������������
�����к���ȩ���������������м�������������Һ��ˮԡ���ȣ���������������֤������ȩ������������ ��
��
(4)���������������У��ҷ����к��е��Ȼ�������������ʣ����Ա�̼��ǿ����ʹʯ����Һ��Ϊ��ɫ�������еķ��ǻ�����NaOH������Ӧ�����ݱ����׳�ʯ̿�ᣬ�������ԣ�����ʹʯ����Һ��ɫ�����������еĴ��ǻ�Ϊ���Ի��ţ����������ԣ�����������ǿ������˳��������>��>����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ������£�NCl3��һ����״Һ�壬����ӿռ乹����NH3���ƣ����ж�NCl3��NH3���й�������ȷ����( )
A. ������N��Cl��������CCl4������C��Cl���������
B. NCl3�����ǷǼ��Է���
C. NBr3��NCl3�ӷ�
D. �ڰ�ˮ�У���NH3��H2O�����(����������ʾ)����γ�NH3��H2O���ӣ���NH3��H2O�ĽṹʽΪ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������0.1mol��L��1 Na2CO3��Һ������������ǣ� ��
A.0.5 L����Һ�У�Na+�����ʵ���Ũ��Ϊ0.2mol��L��1
B.1 L����Һ�У���CO32������ĿС��0.1NA(NA�ǰ����ӵ�����)
C.��1 L����Һ��ȡ��100 mL����ȡ������Һ��Na2CO3�����ʵ���Ũ��Ϊ0.01mol��L-1
D.ȡ����Һ10 mL����ˮϡ����100 mL��Na2CO3�����ʵ���Ũ��Ϊ0.01mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��д��SO3���ӵĿռ乹����____����____���ӣ����������������Ǽ��������������ĵȵ�����Ļ�ѧʽ��һ��������______ (д��һ��)�����ǵ�����ԭ�Ӳ��õ��ӻ���ʽ����_______��
��2����ȩ(H2C��O)��Ni�������¼���ɵü״�(CH3OH)���״�������Cԭ�ӵ��ӻ���ʽΪ__���״������ڵ�O��C��H����___����������������������������ȩ�����ڵ�O��C��H���ǣ��״���������ˮ������Ҫԭ����_____��
��3����֪�ߵ�����������ʽ����ѧʽ�ֱ�ΪH5IO6��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____��������������������������HIO4��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʽΪC3H7Br���л���������˵��������ܷ�������һϵ��ת����

(1)��B�ܷ���������Ӧ����ش��������⣺
����ȷ���л���Ľṹ��ʽ��__________________��
���û�ѧ����ʽ��ʾ����ת�����̣�
�ף�NaOH![]() ________________________________________________________��
________________________________________________________��
B��Ag(NH3)2OH�D��_______________________________________________________��
(2)��B���ܷ���������Ӧ����ش��������⣺
����ȷ��A�Ľṹ��ʽ��_______________________________________________________��
���û�ѧ����ʽ��ʾ����ת�����̣�
�ף�NaOH![]() __________________________________________________��
__________________________________________________��
A�D��B��_________________________________________________��
D�D��E��___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ��ķ�Ӧԭ�������ӷ���ʽ��ʾ��ȷ���ǣ� ��
A. �����£�����Ȼ����ҺpH<7��֤��һˮ�ϰ��������NH4����2H2O=NH3��H2O��H3O��
B. ������������Һ��ȥþ���е���������2Al��2OH����2H2O=2AlO2����3H2��
C. ��̼��������Һ����ˮ�����е��Ȼ���
 +2HCO3-��
+2HCO3-��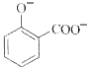 +2H2O+2CO2��
+2H2O+2CO2��
D. �ø�����ر���Һ�ζ����2MnO4����16H����5C2O42��=2Mn2����10CO2����8H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�� A��ʯ���ѽ�������Ҫ�ɷ���A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ��D�dz����������е�ζƷ����ش��������⣺
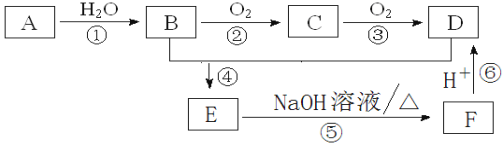
��1��д��C�Ľṹ��ʽ________________________��
��2��д�����з�Ӧ�ķ�Ӧ���ͣ� ��________________����________________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��______________________________________________________________��
��______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ������������
��1�����������ӣ�Fe3�������Լ�____________���ѧʽ��������______________���������ӣ�Fe3�����Ļ����¼����Ƿ����������ӣ�Fe2�������Լ���_________���ѧʽ��������_______________��
��2����С�մ�Ƭ����θ���������ӷ���ʽΪ____________��
��3��þ���Ż�ʱ��������Һ̬CO2����������ԭ����_____________�����û�ѧ����ʽ��ʾ����
��4����ȥ����Fe2O3��ĩ������Al2O3�������ӷ���ʽΪ______________��
��5����AlCl3��Һ���ɣ����գ��õ��Ĺ��������__________��AlCl3��Һ��NaHCO3��Һ���ʱ������Ӧ�����ӷ���ʽΪ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��CH3��CH![]() CH2 + HBr
CH2 + HBr![]() CH3��CHBr��CH3(��Ҫ����)��1 molij��A���ȼ�պ�õ�8molCO2��4molH2O����A�ڲ�ͬ�������ܷ�������ͼ��ʾ��һϵ�б仯��
CH3��CHBr��CH3(��Ҫ����)��1 molij��A���ȼ�պ�õ�8molCO2��4molH2O����A�ڲ�ͬ�������ܷ�������ͼ��ʾ��һϵ�б仯��
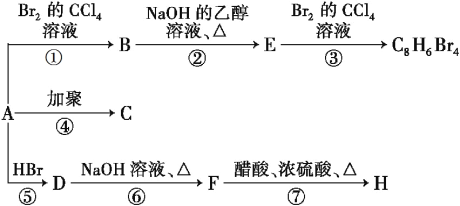
��1��A�Ľṹ��ʽ��_______��
��2��������Ӧ����_______��Ӧ������_______��Ӧ��(�Ӧ����)
��3��д��C��H���ʵĽṹ��ʽ��C_________��H_________��
��4��д��D![]() F��Ӧ�Ļ�ѧ����ʽ_______________��
F��Ӧ�Ļ�ѧ����ʽ_______________��
��5��������G��F��ͬ���칹�壬��FeCl3��Һ����ɫ���ұ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�ӣ�д������������G�Ľṹ��ʽ__________������дһ�ּ��ɣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com