
����Ŀ������������Ԫ��X��Y��Z��R��T��ԭ�Ӱ뾶��ԭ��������ϵ��ͼ��ʾ��Rԭ�������������ǵ��Ӳ�����2����Y��Z���γ�Z2Y��Z2Y2�����ӻ����Z��T���γɻ�����Z2T�������ƶ���ȷ���ǣ� ��

A.�����Ӱ뾶��T>Z>Y
B.Z2Y��Z2Y2�����еĻ�ѧ��������ͬ
C.����X2Y�ķе����X2T�����Ƴ�X2Y���ȶ���ǿ��X2T
D.ZXT��ˮ��Һ�������ԣ��ٽ���ˮ�ĵ���
���𰸡�D
��������
Rԭ�������������ǵ��Ӳ�����2����R����ΪCԪ�ػ�SԪ�أ�����ͼʾԭ�Ӱ뾶��ԭ��������ϵ��֪RӦΪCԪ�أ�Y��Z���γ�Z2Y��Z2Y2�����ӻ����ӦΪNa2O��Na2O2����YΪOԪ�أ�ZΪNaԪ�أ�Z��T�γɵ�Z2T�������T��ԭ�Ӱ뾶��NaС��ԭ������T>Z����TӦΪSԪ�أ�X��ԭ�Ӱ뾶��С��ԭ��������С��ԭ�Ӱ뾶Ҳ��С����XӦΪHԪ�أ���϶�Ӧ���ʡ�������������Լ���ĿҪ������⡣
���������ƶϿ�֪��X��HԪ�أ�Y��OԪ�أ�Z��NaԪ�أ�R��CԪ�أ�T��SԪ�ء�
A.S2-������3�����Ӳ㣬O2-��Na+������2�����Ӳ㣬�������Ӻ�����Ӳ���Խ�࣬���Ӱ뾶Խ�����Ӻ�����Ӳ�����ͬʱ���˵����Խ�����Ӱ뾶ԽС���������Ӱ뾶��T>Y>Z��A����
B. Z2Y��Z2Y2��ʾ����Na2O��Na2O2��Na2Oֻ�����Ӽ���Na2O2�������Ӽ������Թ��ۼ�����������Ļ�ѧ�����Ͳ���ͬ��B����
C.O��S��ͬһ�����Ԫ�أ�����H2O����֮������������H2S����֮��ֻ���ڷ��»���������Ĵ���ʹH2O�ķе��H2S�ߣ������ʵ��ȶ���������ڵĹ��ۼ���ǿ���йأ�����Ӽ���������С�أ�C����
D.ZXT��ʾ����NaHS��������Ϊǿ�������Σ�����Һ��HS-ˮ�⣬����ˮ���������H+����γ�H2S��ʹˮ�ĵ���ƽ�������ƶ����ٽ���ˮ�ĵ��룬ʹ��Һ��c(OH-)�������մﵽƽ��ʱ����Һ��c(OH-)>c(H+)����Һ�Լ��ԣ�D��ȷ��
�ʺ���ѡ����D��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ұ��ҵ�����Ṥҵ�ķ�����Һ�к�����������Ⱦ���أ����봦����������ŷš�
��.�û���̿��������β���еĵ������
(1)��֪:��4NH3(g)+5O2(g)=4NO(g)+6H2O(l)����H1=a kJ��mol-1
��4NH3(g)+3O2(g)=2N2(g)+6H2O(l)����H2=b kJ��mol-1
��C(s)+O2(g)=CO2(g)����H3=c kJ��mol-1
��ӦC(s)+2NO(g) ![]() N2(g)+CO2(g)����H=_____��
N2(g)+CO2(g)����H=_____��
(2)���ݻ�������ܱ������У�һ������NO��������C������Ӧ��C(s)+2NO(g) ![]() N2(g)+CO2(g)����H=Q kJ��mol-1��ƽ��ʱc(NO)���¶�T�Ĺ�ϵ��ͼ1��ʾ������˵����ȷ����______��
N2(g)+CO2(g)����H=Q kJ��mol-1��ƽ��ʱc(NO)���¶�T�Ĺ�ϵ��ͼ1��ʾ������˵����ȷ����______��

A.�����������䣬�ı����̿��������ƽ��һ�����ƶ�
B.�÷�Ӧ��Q��0������T1��T2��T3��Ӧ��ƽ�ⳣ��:K1��K2��K2
C.�¶�ΪT2ʱ������Ӧ��ϵ����״̬D�����ʱv����v��
D.��״̬B��C��D��ϵ��ѹǿ�ֱ�Ϊp(B)��p(C)��p(D)����p(D)=p(C)��p(B)
(3)��֪ij�¶�ʱ����ӦC(s)+2NO(g) ![]() N2(g)+CO2(g)��ƽ�ⳣ��K=9/16���ڸ��¶��µ�2 L�ܱ�������Ͷ�������Ļ���̿��2.0 mol NO������Ӧ��t1ʱ�̴ﵽƽ�⣬����ͼ2�л�����Ӧ������c(NO)��ʱ��t�ı仯���ߡ�_______
N2(g)+CO2(g)��ƽ�ⳣ��K=9/16���ڸ��¶��µ�2 L�ܱ�������Ͷ�������Ļ���̿��2.0 mol NO������Ӧ��t1ʱ�̴ﵽƽ�⣬����ͼ2�л�����Ӧ������c(NO)��ʱ��t�ı仯���ߡ�_______
(4)��ҵ��ʵ�ʴ�������ʱ�����û���̿����������NH3��ԭNO��ͬʱͨ��һ������O2����ߴ���Ч������n(NH3)=n(NO)ʱ��д����ϵ���ܷ�Ӧ�Ļ�ѧ����ʽ:______________��
��.���������ۻ��ⷨ������ˮ�е������Ρ�
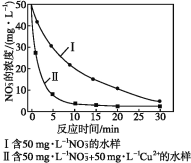
(5)�������۴�����ˮ��NO3-�����ӷ���ʽΪ4Fe+ NO3-+10H+=4Fe2++NH4++3H2O��
ʵ��֤ʵ��pHƫС���ᵼ��NO3-��ȥ�����½�����ԭ����_______________����ͬ�����£���������ȥ����ͬˮ����NO3-�������нϴ����(��ͼ)�������ò���Ŀ���ԭ����__��
(6)��ⷨ����ˮ�������ε�ԭ�����Խ���Pt���缫�������ӽ���Ĥ����Һ��Ϊ������������������Ϊ�������εĹ�ҵ��ˮ����ֱͨ����Դ���е�⡣��д�������ĵ缫��Ӧʽ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮΪ�ܼ������к͵ζ���ԭ���ǣ�H3O++OH-��2H2O����֪Һ̬SO2�ʹ�ˮ�ĵ������������ΪҺ̬SO2Ҳ�ᷢ������⣺SO2��l��+SO2��l��![]() SO32-+SO2+������Һ̬SO2Ϊ�ܼ�����SOCl2�ζ�Cs2SO3������������������� �� ��
SO32-+SO2+������Һ̬SO2Ϊ�ܼ�����SOCl2�ζ�Cs2SO3������������������� �� ��
A. �õζ���Ӧ���Ա�ʾΪ��SO32-+ SO2+��2SO2
B. ��һ���¶��£�Һ̬SO2��c��SO32-����c��SO2+���ij˻���һ������
C. �����Ĵ��ڣ�˵��SO2�����ӻ�����
D. ��ͨ�����������仯���ж��Ƿ�ζ��յ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯�����ڹ�ũҵ������������������Ҫ���á�
��1����T��ʱ����0��6molH2��0��4molN2�����ݻ�Ϊ2 L���ܱ�������(ѹǿΪmPa)������Ӧ��3H2+N2![]() 2NH3��H<0���������¶Ȳ��䣬ij��ȤС��ͬѧ��÷�Ӧ������������ѹǿ��ʱ��仯��ͼ��ʾ��8 min�ڷ���NH3��ƽ����������Ϊ___mol��L-1��min-1��
2NH3��H<0���������¶Ȳ��䣬ij��ȤС��ͬѧ��÷�Ӧ������������ѹǿ��ʱ��仯��ͼ��ʾ��8 min�ڷ���NH3��ƽ����������Ϊ___mol��L-1��min-1��

��2������T��ʱ����0��6molH2��0��4molN2����һ�ݻ��ɱ���ܱ��� ���С�
�����и�����˵���÷�Ӧ�Ѵﵽƽ��״̬����______(�����)��
a��������N2��H2��NH3��Ũ��֮��Ϊl��3��2
b��3v��(N2)=v��(H2)
c��3v��(H2)=2v��(NH3)
d�����������ܶȱ��ֲ���
e�������������ƽ����Է�����������ʱ����仯
���������´ﵽƽ��ʱNH3������������⣨1��������NH3������������________(�������������С������������)��
���ﵽƽ��ı�ijһ����ʹ��Ӧ���ʷ�������ͼ��ʾ�ı仯���� �������������_________��

a�������¶ȣ�ͬʱ��ѹ
b�������¶ȣ�ͬʱ��ѹ
c�������¶ȡ�ѹǿ���䣬����Ӧ��Ũ��
d�������¶ȡ�ѹǿ���䣬��С������Ũ��
��3�����᳧��β�����е��������������ֱ���ŷŽ���Ⱦ������ �����ܽ��������ﻹԭΪ������ˮ���䷴Ӧ����Ϊ��
2NH3(g)+5NO2(g)=7NO(g)+3H2O(g)![]() H=-akJ��mol-1
H=-akJ��mol-1
4NH3(g)+6NO(g)=5N2(g)+6H2O(g)![]() H=-bkJ��mol-1
H=-bkJ��mol-1
��NH3ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ��______________��
����״����NO��NO2�������40��32L��������ˮ��ȫ���գ�������״���µ���42��56L���û��������NO��NO2�����֮��Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£���һ�̶�������ܱ������У���˵�����淴ӦA (g)��3B (g)![]() 2C (g)�ﵽƽ���˵���У���ȷ�������
2C (g)�ﵽƽ���˵���У���ȷ�������
��C ���������ʺ� C �ķֽ��������
����λʱ�������� a mol A��ͬʱ���� 3a mol B
�������ܶȲ��ٱ仯
�� ��������ƽ����Է�����������
��A��B��C �����ʵ���֮��Ϊ 1��3��2
A.�٢�B.�ڢۢ�C.�٢ܢ�D.�ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ܱ������������·�Ӧ��aX��g��+bY��g��![]() nW��g��.ij��ѧ��ȤС���ͬѧ���ݴ˷�Ӧ�ڲ�ͬ�����µ�ʵ�����ݣ���������������ͼ��
nW��g��.ij��ѧ��ȤС���ͬѧ���ݴ˷�Ӧ�ڲ�ͬ�����µ�ʵ�����ݣ���������������ͼ��

���У��أ�W����ʾW�ڷ�Ӧ������е����������t��ʾ��Ӧʱ�䣮��������������ʱ�����з�����ȷ����
A. ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��p2��p1��a+b��n
B. ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��p1��p2��n��a+b
C. ͼ���������ͬ��ͬѹ�´����Է�Ӧ��Ӱ�죬��1ʹ�õĴ���Ч����
D. ͼ������Dz�ͬ�¶ȶԷ�Ӧ��Ӱ�죬��T1��T2����H��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
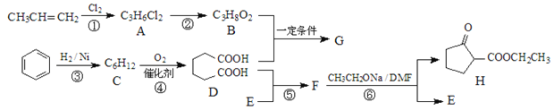
����Ŀ���������ܼ�G��ijҽҩ�м���H��һ�ֺϳ�·����ͼ�����ַ�Ӧ������ȥ����
��֪��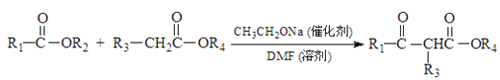 +R2OH
+R2OH
(1)A��������___________________��
(2)д�����з�Ӧ�ķ�Ӧ���ͣ���Ӧ����_____________����Ӧ����__________��
(3)G�Ľṹ��ʽΪ_____________________��F�ķ���ʽΪ_____________________��
(4)д����Ӧ���Ļ�ѧ����ʽ____________________��
(5)C���ڶ���ͬ���칹�壬д���˴Ź�������ֻ�����ַ��ͬ���칹��Ľṹ��ʽ��
____________��
(6)����һ���Լ��Ϳ��Լ���B��D��H�����Լ���____________��
(7)�������Ϻϳ�·�ߵ���Ϣ���Լױ����Ҵ����Ҵ���Ϊԭ�Ϻϳ������л���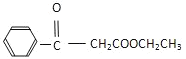 �����Լ���ѡ��___________��
�����Լ���ѡ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ʒ������Ϊ������������������ҽҩ���ܼ����ϳ�a����Ʒ��G��·��֮һ���£�

��֪��RCOOC2H5![]()

��ش��������⣺
�� A���������ŵ�������________________��
�� A���⻯��Z��C7H12O3����д��Z��һ�������¾ۺϷ�Ӧ�Ļ�ѧ����ʽ��
_______________________________________________________________��
�� B�ķ���ʽΪ_________��д��ͬʱ��������������B����״ͬ���칹��Ľṹ��ʽ��_____________��
�� �˴Ź���������2�����շ� �� �ܷ���������Ӧ
(4) B �� C��E �� F�ķ�Ӧ���ͷֱ�Ϊ_____________��_____________��
�� C �� D�Ļ�ѧ����ʽΪ____________________________________________��
�� �Լ�Y�Ľṹ��ʽΪ______________________��
�� ͨ�������µķ�Ӧ������E��F��G���Լ���______________��_____________��
�� G��H2O���ӳɵò�������̼ԭ�ӣ�����4����ͬԭ�ӻ�ԭ���ŵ�̼ԭ�ӽ�����̼ԭ�ӣ��Ļ�����H��д��H�Ľṹ��ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ�����A��B�����2 L���ܱ������У��������з�Ӧ��3A(g)��B(g)xC(g)��2D(g)����2 min����D��Ũ��Ϊ0.5 mol��L-1��c(A)��c(B)��3��5����C��ʾ��ƽ������v(C)��0.25 mol��L-1��min-1������˵����ȷ����(�� ��)
A. �÷�Ӧ����ʽ�У�x��1 B. 2 minʱ��A�����ʵ���Ϊ0.75 mol
C. 2 minʱ��A��ת����Ϊ50% D. ��Ӧ����v(B)��0.25 mol��L-1��min-1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com