
����Ŀ����Ʒ������Ϊ������������������ҽҩ���ܼ����ϳ�a����Ʒ��G��·��֮һ���£�

��֪��RCOOC2H5![]()

��ش��������⣺
�� A���������ŵ�������________________��
�� A���⻯��Z��C7H12O3����д��Z��һ�������¾ۺϷ�Ӧ�Ļ�ѧ����ʽ��
_______________________________________________________________��
�� B�ķ���ʽΪ_________��д��ͬʱ��������������B����״ͬ���칹��Ľṹ��ʽ��_____________��
�� �˴Ź���������2�����շ� �� �ܷ���������Ӧ
(4) B �� C��E �� F�ķ�Ӧ���ͷֱ�Ϊ_____________��_____________��
�� C �� D�Ļ�ѧ����ʽΪ____________________________________________��
�� �Լ�Y�Ľṹ��ʽΪ______________________��
�� ͨ�������µķ�Ӧ������E��F��G���Լ���______________��_____________��
�� G��H2O���ӳɵò�������̼ԭ�ӣ�����4����ͬԭ�ӻ�ԭ���ŵ�̼ԭ�ӽ�����̼ԭ�ӣ��Ļ�����H��д��H�Ľṹ��ʽ��________________________��
���𰸡��ʻ����Ȼ� ![]() C8H14O3
C8H14O3 ![]() ȡ����Ӧ ������Ӧ��ȡ����Ӧ��
ȡ����Ӧ ������Ӧ��ȡ����Ӧ�� 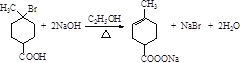 CH3MgX��X=Cl��Br��I�� NaHCO3��Һ Na�����������𰸾��ɣ�
CH3MgX��X=Cl��Br��I�� NaHCO3��Һ Na�����������𰸾��ɣ� 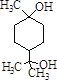
��������
A��һ��������ת����B��B��HBr����ȡ����Ӧ����CΪ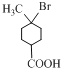 ��C������ȥ��Ӧ����D����D�Ľṹ��ʽΪ��
��C������ȥ��Ӧ����D����D�Ľṹ��ʽΪ��![]() ��D�ữ�õ�E����E�Ľṹ��ʽΪ
��D�ữ�õ�E����E�Ľṹ��ʽΪ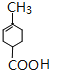 ��E���Ҵ�����������Ӧ����FΪ
��E���Ҵ�����������Ӧ����FΪ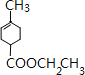 ��F��һ�������·�Ӧ����G���ݴ˽��н��
��F��һ�������·�Ӧ����G���ݴ˽��н��
��1������A�Ľṹ��ʽ��A�������Ĺ�����Ϊ���ʻ����Ȼ���
��2��A���⻯�õ����ǣ�![]() ��Z�ܷ������۷�Ӧ��
��Z�ܷ������۷�Ӧ��![]() ��
��
��3������B�Ľṹ��ʽΪ��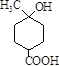 �������ʽΪ��C8H14O3��B��ͬ���칹���з����������ǣ�
�������ʽΪ��C8H14O3��B��ͬ���칹���з����������ǣ�![]() ��
��
��4��Bת��ΪC�ķ�ӦΪȡ����Ӧ��Eת��ΪF��������Ӧ��Ҳ����ȡ����Ӧ��
��5������ת����ϵ��Cת��ΪD�ķ�ӦΪ�� ��
��
��6�������������֪��Ϣ����֪Y�Լ�Ϊ��CH3MgX��X=Cl��Br��I����
��7������E��F��G�����ʵĽṹ��ʽ��Ҫ��������ѡȡNaHCO3��Һ�ͽ���Na���ɣ�
��8��G��ˮ�ӳ����õIJ�������̼ԭ�ӵ������ǣ�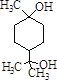 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ϳɰ���ҵ�е�ԭ������ȡ�뾻������Ҫ�Ļ��ڡ�
��1����֪�йط�Ӧ�������仯����ͼ��

д��CH4(g)��H2O(g)��Ӧ��ȡCO(g)��H2(g)���Ȼ�ѧ����ʽ�� ___________________��
��2����CH4-CO2������������������CH4(g)��CO2(g)![]() 2CO (g)��2H2(g)����H
2CO (g)��2H2(g)����H
�������Եõ��ϳ�����CO��H2����������������ļ��ž�����Ҫ���塣������Ӧ����صĻ�ѧ�������������±���ʾ���ٸ��ݼ��ܼ��㣬�÷�Ӧ�Ħ�H��_______kJ��mol��1��

�ڰ�һ������ȼ���CH4��CO2���ں�ѹ�·�����Ӧ���¶ȶ�CO��H2���ʵ�Ӱ����ͼ��ʾ��ʵ�������д˷�Ӧ��ѡ�¶�Ϊ900�棬ԭ����_______________________��

��ij�¶��£���1 mol CH4��2 mol CO2�Լ��������м���2 L�����У��ﵽƽ��ʱ��(CH4)��50%��ƽ�ⳣ��K=____mol2��L��2��
��3���ϳ����ڽ���ϳ���ǰ���ô��������ͭ(��)��Һ���������е�CO���ʣ��䷴Ӧ�ǣ�Cu(NH3)2(CH3COO)��CO��NH3![]() Cu(NH3)3(CH3COO)��CO����H��0
Cu(NH3)3(CH3COO)��CO����H��0
�����ȥԭ������CO��ԭ����_______________________��
���������ͭ(��)����CO��������������Ӧ��__________������ţ���
A�����¸�ѹ B�����µ�ѹ C�����¸�ѹ D�����µ�ѹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��X��Y��Z��R��T��ԭ�Ӱ뾶��ԭ��������ϵ��ͼ��ʾ��Rԭ�������������ǵ��Ӳ�����2����Y��Z���γ�Z2Y��Z2Y2�����ӻ����Z��T���γɻ�����Z2T�������ƶ���ȷ���ǣ� ��

A.�����Ӱ뾶��T>Z>Y
B.Z2Y��Z2Y2�����еĻ�ѧ��������ͬ
C.����X2Y�ķе����X2T�����Ƴ�X2Y���ȶ���ǿ��X2T
D.ZXT��ˮ��Һ�������ԣ��ٽ���ˮ�ĵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ǰ�����ڵ���������Ԫ�أ�
X | ������ˮ��Ӧ���ɵ������ʹ�����ǵ�ľ����ȼ |
Y | �����������ܼ��������ܼ��ĵ�������� |
Z | �����������Ǵ�����3�� |
W | ��Ԫ�����ڱ��д��ڵ�8�� |
R | �������ռ��9���������2��δ�ɶԵ��� |
Q | �ڶ������е���������� |
��1��XԪ������_____________��RZ3��R���ӻ���ʽΪ_____________��
��2��Wԭ�ӽṹʾ��ͼΪ_____________��,Y��Z��Q�縺����С����˳��_____________��(��Ԫ�ط��ű�ʾ��
��3����Q�⻯��Ũ��Һ�м���Cu�ۣ���ͨ��Z2����ַ�Ӧ����Һ������ɫ���÷�Ӧ�����ӷ���ʽΪ_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ǵ����Ϻ�����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á���ش��������⣺��ͼ���漰����Ϊ��̬��

��1����ͼ��1mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ______��
��2����0.5L���ܱ������У�һ�����ĵ����������������»�ѧ��Ӧ��N2��g��+3H2��g��![]() 2NH3��g����H��0���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
2NH3��g����H��0���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
t/�� | 200 | 300 | 400 |
K | K1 | K2 | 0.5 |
������������⣺
���ԱȽ�K1��K2�Ĵ�С��K1______K2����д����������=��������������
�����и�������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������_______���������ĸ����
a .������N2��H2��NH3��Ũ��֮��Ϊ1��3��2 b. ����N2����=3����H2����
c .������ѹǿ���ֲ��� d. ���������ܶȱ��ֲ���
����400��ʱ�������NH3��N2��H2�����ʵ����ֱ�Ϊ1mol��2mol��3molʱ����÷�Ӧ������N2����______����N2��������д��������=��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪NA�ǰ����ӵ�������ֵ������˵��������ǣ� ��
A.3g3He���е�������Ϊ1NA
B.48g�������10g�춡��Ļ�����й��ۼ���ĿΪ13NA
C.�ڱ�״���£�22.4LNH3��O2��ȫ����ΪNOʱ��ת�Ƶ��ӵ���ĿΪ5NA
D.һ�������£�6.4gͭ���������Ӧ��ת�Ƶ�����Ϊ0.2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F��Ϊ���ڱ���ǰ������Ԫ�أ���ԭ������������������A��B��CΪ�����ڷǽ���Ԫ�ء�A���γɻ�������������Ԫ�أ�Bԭ�ӻ�̬�����Ų���ֻ��һ��δ�ɶԵ��ӣ�C��ͬ����Ԫ����ԭ�Ӱ뾶��С��Ԫ�أ�D�Ļ�̬ԭ����ǰ������Ԫ�صĻ�̬ԭ���е���������ࣻE��D���ڣ�E��ij��������X��C���⻯���Ũ��Һ����ʱ��Ӧ������ʵ������ȡ��̬����C��F��D��������������ȡ�
�ش���������(��ػش����Ԫ�ط��ű�ʾ)��
(1)D�Ļ�̬ԭ�ӵĺ�������Ų�ʽ��______________��
(2)B���⻯��ķе��C���⻯��ķе�________(����������������)��ԭ����______________________��
(3)A�ĵ縺��________(��������������С����)C�ĵ縺�ԣ�A�γɵ��⻯��A2H4��A���ӻ�������________��
(4) X����ȡC�����е�������________��C��ij�ֺ������γ�����ʵ��������ȡ����������������л�ѧ���ļ���________(����>������������<��)109��28����

(5)��֪F��C��ij�ֻ�����ľ����ṹ��ͼ��ʾ����û�����Ļ�ѧʽ��__________________����F��Cԭ������ľ���Ϊa cm����þ�����ܶ�Ϊ________g��cm��3(ֻҪ������ʽ�����ؼ������ֵ�������ӵ���������ֵΪNA)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܱ������ͨ��x mol H2(g)��y mol ��2(g)��������ӦH2(g)+I2(g) ![]() 2HI(g) ��H<0���ı�������������Ӧ���ʽ���θı�?(��������������С������������)
2HI(g) ��H<0���ı�������������Ӧ���ʽ���θı�?(��������������С������������)
��1�������¶�____________��
��2���������__________��
��3����������H2_________________��
��4���������������___________________��
��5�������ݻ����䣬ͨ������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£����ܵ���ʵı�����Һ�д����ų����ܽ�ƽ�⣬��֪��
���� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
Ksp(25 ��) | 8.0��10��16 | 2.2��10��20 | 4.0��10��38 |
25 ��ʱ�����ں�Fe2(SO4)3��FeSO4��CuSO4��0.5 mol��1 L�����Һ�������ϱ������жϣ�����˵������ȷ����(����)
A. ��pH��5����Һ�У�Fe3�����ܴ�������
B. �����Һ��c(SO![]() )��[c(Fe3��)��c(Fe2��)��c(Cu2��)]>5��4
)��[c(Fe3��)��c(Fe2��)��c(Cu2��)]>5��4
C. ������Һ����μ���0.1 mol��L��1NaOH��Һ�����ȿ������ɫ����
D. ������Һ�м���������ˮ��������pH��3��4���ˣ��ɻ�ϴ�����CuSO4��Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com