
ΓΨΧβΡΩΓΩΘ®1Θ© “Έ¬œ¬Θ§2g±ΫΘ®C6H6Θ©Άξ»Ϊ»Φ…’…ζ≥…“ΚΧ§Υ°ΚΆCO2Θ§Ζ≈≥ω83.6kJΒΡ»»ΝΩΘ§–¥≥ω1molC6H6Άξ»Ϊ»Φ…’ΒΡ»»Μ·―ßΖΫ≥Χ ΫΘΚ______ΓΘ
Θ®2Θ©“―÷ΣΘΚFe2O3Θ®sΘ©+![]() CΘ®sΘ©=
CΘ®sΘ©=![]() CO2Θ®gΘ©+2FeΘ®sΘ©ΓςH=+akJmol-1ΘΜCΘ®sΘ©+O2Θ®gΘ©=CO2Θ®gΘ©ΓςH=-bkJmol-1Θ§‘ρ2FeΘ®sΘ©+
CO2Θ®gΘ©+2FeΘ®sΘ©ΓςH=+akJmol-1ΘΜCΘ®sΘ©+O2Θ®gΘ©=CO2Θ®gΘ©ΓςH=-bkJmol-1Θ§‘ρ2FeΘ®sΘ©+![]() O2Θ®gΘ©=Fe2O3Θ®sΘ©ΒΡΓςH=________ΓΘ
O2Θ®gΘ©=Fe2O3Θ®sΘ©ΒΡΓςH=________ΓΘ
Θ®3Θ©“―÷ΣΦΗ÷÷Μ·―ßΦϋΒΡΦϋΡή»γ±μΥυ ΨΘΚ
Μ·―ßΦϋ | ClΓΣCl | FΓΣF | ClΓΣF |
ΦϋΡή/ kJΓΛmolΓΣ1 | 242 | 159 | 172 |
‘ρΖ¥”ΠCl2(g)+3F2(g)![]() 2ClF3(g)ΒΡΓςH=_____________ kJΓΛmol-1ΓΘ
2ClF3(g)ΒΡΓςH=_____________ kJΓΛmol-1ΓΘ
Θ®4Θ©»γΆΦ «““ΆιΓΔΕΰΦΉΟ―»Φ…’Ιΐ≥Χ÷–ΒΡΡήΝΩ±δΜ·ΆΦΓΘ
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
ΔΌ““ΆιΒΡ»Φ…’»»ΠΛH=_______kJΓΛmol-1ΓΘ
ΔΎΗυΨίΧβΆΦ–¥≥ωΕΰΦΉΟ―Άξ»Ϊ»Φ…’ ±ΒΡ»»Μ·―ßΖΫ≥Χ Ϋ__________ΓΘ
ΓΨ¥πΑΗΓΩC6H6Θ®lΘ©+![]() O2Θ®gΘ©=6CO2Θ®gΘ©+6H2OΘ®lΘ©ΓςH=-3260.4kJ/mol Θ®-
O2Θ®gΘ©=6CO2Θ®gΘ©+6H2OΘ®lΘ©ΓςH=-3260.4kJ/mol Θ®-![]() b-aΘ©kJmol-1Μρ-Θ®
b-aΘ©kJmol-1Μρ-Θ®![]() b+aΘ©kJmol-1 -313 -1560 CH3OCH3(g)+3O2(g)=2CO2(g)+3H2O(l) ΠΛH=-1455 kJ/mol
b+aΘ©kJmol-1 -313 -1560 CH3OCH3(g)+3O2(g)=2CO2(g)+3H2O(l) ΠΛH=-1455 kJ/mol
ΓΨΫβΈωΓΩ
(1)2g“ΚΧ§±Ϋ(C6H6)Έο÷ ΒΡΝΩ=![]() =
=![]() molΘΜΆξ»Ϊ»Φ…’…ζ≥…“ΚΧ§Υ°ΚΆCO2Θ§Ζ≈≥ω83.6kJΒΡ»»ΝΩΘ§1mol±Ϋ»Φ…’Ζ≈»»=83.6kJΓΝ39mol=3260.4kJΘΜΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣΘΚC6H6(l)+
molΘΜΆξ»Ϊ»Φ…’…ζ≥…“ΚΧ§Υ°ΚΆCO2Θ§Ζ≈≥ω83.6kJΒΡ»»ΝΩΘ§1mol±Ϋ»Φ…’Ζ≈»»=83.6kJΓΝ39mol=3260.4kJΘΜΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣΘΚC6H6(l)+![]() O2(g)ΘΫ6CO2(g)+6H2O(l)ΓςH=-3260.4kJ/molΘΜ
O2(g)ΘΫ6CO2(g)+6H2O(l)ΓςH=-3260.4kJ/molΘΜ
(2)ΔΌFe2O3Θ®sΘ©+![]() CΘ®sΘ©=
CΘ®sΘ©=![]() CO2Θ®gΘ©+2FeΘ®sΘ©ΓςH=+akJmol-1
CO2Θ®gΘ©+2FeΘ®sΘ©ΓςH=+akJmol-1
ΔΎCΘ®sΘ©+O2Θ®gΘ©=CO2Θ®gΘ©ΓςH=-bkJmol-1
ΗυΨίΗ«ΥΙΕ®¬…Θ§‘ρ2Fe(s)+![]() O2(g)ΘΫFe2O3(s)Ω…“‘ΗυΨί
O2(g)ΘΫFe2O3(s)Ω…“‘ΗυΨί![]() ΓΝΔΎ-ΔΌΒΟΒΫΘ§“ρ¥Υ2Fe(s)+
ΓΝΔΎ-ΔΌΒΟΒΫΘ§“ρ¥Υ2Fe(s)+![]() O2(g)ΘΫFe2O3(s)ΒΡΓςH=-
O2(g)ΘΫFe2O3(s)ΒΡΓςH=-![]() b-akJmol-1Μρ-(
b-akJmol-1Μρ-(![]() b+a)kJmol-1ΘΜ
b+a)kJmol-1ΘΜ
(3)Ζ¥”Πλ ±δΓςH=Ζ¥”ΠΈοΉήΦϋΡή-…ζ≥…ΈοΉήΦϋΡήΘ§Ζ¥”ΠCl2(g)+3F2(g)![]() 2ClF3(g)ΒΡΓςH=242kJ/mol+3ΓΝ159kJ/mol-2ΓΝ3ΓΝ172kJ/mol=-313kJ/molΘΜ
2ClF3(g)ΒΡΓςH=242kJ/mol+3ΓΝ159kJ/mol-2ΓΝ3ΓΝ172kJ/mol=-313kJ/molΘΜ
(4)ΔΌ“άΨί‘≠Ή” ΊΚψΖ÷ΈωΩ…÷Σ«β‘≠Ή” ΊΚψΘ§6a=2Θ§a=![]() Θ§‘ρΗυΨίΆΦœσΖ÷ΈωΩ…÷Σ
Θ§‘ρΗυΨίΆΦœσΖ÷ΈωΩ…÷Σ![]() mol““ΆιΆξ»Ϊ»Φ…’Ζ≈»»520kJΘ§Υυ“‘1mol““ΆιΆξ»Ϊ»Φ…’Ζ≈»»ΈΣ520kJΓΝ3=1560kJΘ§‘ρ““ΆιΒΡ»Φ…’»»ΓςH=-1560kJΓΛmol-1ΘΜ
mol““ΆιΆξ»Ϊ»Φ…’Ζ≈»»520kJΘ§Υυ“‘1mol““ΆιΆξ»Ϊ»Φ…’Ζ≈»»ΈΣ520kJΓΝ3=1560kJΘ§‘ρ““ΆιΒΡ»Φ…’»»ΓςH=-1560kJΓΛmol-1ΘΜ
ΔΎΗυΨίΆΦœσΖ÷ΈωΩ…÷Σ![]() molΕΰΦΉΟ―Άξ»Ϊ»Φ…’Ζ≈»»485kJΘ§‘ρ1molΕΰΦΉΟ―Άξ»Ϊ»Φ…’Ζ≈»»=485kJΓΝ3=1455kJΘ§Ζ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣΘΚCH3OCH3(g)+3O2(g)ΘΫ2CO2(g)+3H2O(l)ΓςH=-1455 kJmol-1ΓΘ
molΕΰΦΉΟ―Άξ»Ϊ»Φ…’Ζ≈»»485kJΘ§‘ρ1molΕΰΦΉΟ―Άξ»Ϊ»Φ…’Ζ≈»»=485kJΓΝ3=1455kJΘ§Ζ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣΘΚCH3OCH3(g)+3O2(g)ΘΫ2CO2(g)+3H2O(l)ΓςH=-1455 kJmol-1ΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
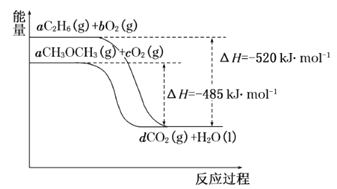
ΓΨΧβΡΩΓΩ(1)≤ΈΩΦΚœ≥…Ζ¥”ΠCO(g)+2H2(g)![]() CH3OH(g)ΒΡΤΫΚβ≥Θ ΐΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
CH3OH(g)ΒΡΤΫΚβ≥Θ ΐΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Έ¬Ε»/Γφ | 0 | 50 | 100 | 200 | 300 | 400 |
ΤΫΚβ≥Θ ΐ | 667 | 100 | 13 | 1.9ΓΝ10-2 | 2.4ΓΝ10-4 | 1ΓΝ10-5 |
ΔΌΗΟΖ¥”Π’ΐΖ¥”Π «___________Θ®ΧνΓΑΖ≈»»Γ±ΜρΓΑΈϋ»»Γ±Θ©Ζ¥”ΠΘΜ
ΔΎ‘ΎTΓφ ±Θ§1LΟή±’»ίΤς÷–Θ§ΆΕ»κ0.1molCOΚΆ0.2molH2Θ§¥οΒΫΤΫΚβ ±Θ§COΉΣΜ·¬ ΈΣ50%Θ§‘ρT=__________ΓφΓΘ
(2)CH3OH“≤Ω…”…CO2ΚΆH2Κœ≥…ΓΘ‘ΎΧεΜΐΈΣ1LΒΡΟή±’»ίΤς÷–Θ§≥δ»κlmolCO2ΚΆ3molH2Θ§“ΜΕ®ΧθΦΰœ¬Ζ¥”ΠΘΚCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ΠΛH=-49.0kJ/molΘ§≤βΒΟCO2ΚΆCH3OH(g)≈®Ε»Υφ ±Φδ±δΜ·»γΆΦΥυ ΨΓΘ
CH3OH(g)+H2O(g) ΠΛH=-49.0kJ/molΘ§≤βΒΟCO2ΚΆCH3OH(g)≈®Ε»Υφ ±Φδ±δΜ·»γΆΦΥυ ΨΓΘ
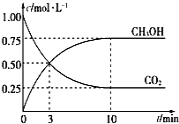
ΔΌΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐ±μ¥ο ΫΈΣK=________ΘΜ¥”Ζ¥”ΠΩΣ ΦΒΫ10minΘ§v(H2)=______molΓΛL-1ΓΛmin-1ΘΜ
ΔΎœ¬Ν–«ιΩωΡήΥΒΟςΗΟΖ¥”Π“ΜΕ®¥οΒΫΤΫΚβΉ¥Χ§ΒΡ «___________(ΧνΉ÷ΡΗ)
A.v(CO2)œϊΚΡ=v(CH3OH)…ζ≥…
B.ΤχΧεΒΡΟήΕ»≤Μ‘ΌΥφ ±ΦδΗΡ±δ
C.CO2ΚΆCH3OHΒΡ≈®Ε»÷°±»≤Μ‘ΌΥφ ±ΦδΗΡ±δ
D.ΤχΧεΒΡΤΫΨυœύΕ‘Ζ÷Ή”÷ ΝΩ≤Μ‘ΌΥφ ±ΦδΗΡ±δ
ΔέΈΣΝΥΦ”ΩλΜ·―ßΖ¥”ΠΥΌ¬ «“ ΙΧεœΒ÷–ΤχΧεΒΡΈο÷ ΒΡΝΩ‘ω¥σΘ§÷ΜΗΡ±δœ¬Ν–Ρ≥“ΜΧθΦΰΘ§Ω…≤…»ΓΒΡ¥κ ©”–___________ (ΧνΉ÷ΡΗ)
A.…ΐΗΏΈ¬Ε» B.Υθ–Γ»ίΤςΧεΜΐ C.‘Ό≥δ»κCO2ΤχΧε D. Ι”ΟΚœ ΒΡ¥ΏΜ·ΦΝ
ΔήœύΆ§Έ¬Ε»œ¬Θ§‘ΎΝμ“ΜΗω»ίΜΐΈΣ1 LΒΡΟή±’»ίΤς÷–≥δ»κ2mol CH3OH(g)ΚΆ2molH2O(g)Θ§¥οΒΫΤΫΚβ ±CO2ΒΡ≈®Ε»____________(ΧνΓΑΘΨΓ±ΓΔΓΑΘΦΓ±ΜρΓΑ=Γ±)0.25molΓΛL-1ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΙΐΕ…Ϋπ τ‘ΣΥΊ―θΜ·ΈοΒΡ”Π”Ο―–ΨΩ «ΡΩ«ΑΩΤ―ß―–ΨΩΒΡ«Α―Ί÷°“ΜΘ§ ‘ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©Εΰ―θΜ·ν―ΉςΙβ¥ΏΜ·ΦΝΡήΫΪΨ” “Έέ»ΨΈοΦΉ»©ΓΔ±ΫΒ»”–ΚΠΤχΧεΩ…ΉΣΜ·ΈΣΕΰ―θΜ·ΧΦΚΆΥ°Θ§¥οΒΫΈόΚΠΜ·ΓΘ”–ΙΊΦΉ»©ΓΔ±ΫΓΔΕΰ―θΜ·ΧΦΦΑΥ°ΥΒΖ®’ΐ»ΖΒΡ «___ΓΘ
A.±Ϋ”κB3N3H6ΜΞΈΣΒ»ΒγΉ”Χε
B.ΦΉ»©ΓΔ±ΫΖ÷Ή”÷–ΧΦ‘≠Ή”Ψυ≤…”Οsp2‘”Μ·
C.±ΫΓΔΕΰ―θΜ·ΧΦ «Ζ«ΦΪ–‘Ζ÷Ή”Θ§Υ°ΚΆΦΉ»© «ΦΪ–‘Ζ÷Ή”
D.Υ°ΒΡΖ–Βψ±»ΦΉ»©ΗΏΒΟΕύΘ§ «“ρΈΣΥ°Ζ÷Ή”ΦδΡή–Έ≥…«βΦϋ
Θ®2Θ©2007Ρξ≈Β±¥ΕϊΈοάμ―ßΫ±ΈΣΖ®ΙζΩΤ―ßΦ“ΑΔΕϊ±¥ΓΛΖ―ΕϊΚΆΒ¬ΙζΩΤ―ßΦ“±ΥΒΟΓΛΗώΝ÷±¥ΗώΕϊΙ≤Ά§ΜώΒΟΘ§“‘±μ’ΟΥϊΟ«‘ΎΨό¥≈ΒγΉη–ß”ΠΘ®CMR–ß”ΠΘ©―–ΨΩΖΫΟφΒΡ≥…ΨΆΓΘΡ≥ΗΤν―–ΆΗ¥Κœ―θΜ·ΈοΘ®»γΆΦ1Θ©Θ§“‘A‘≠Ή”ΈΣΨßΑϊΒΡΕΞΒψΘ§AΈΜΩ…“‘ «CaΓΔSrΓΔBaΜρPbΘ§Β±BΈΜ «VΓΔCrΓΔMnΓΔFe ±Θ§’β÷÷Μ·ΚœΈοΨΏ”–CMR–ß”ΠΓΘ

ΔΌ”ΟAΓΔBΓΔO±μ Ψ’βάύΧΊ βΨßΧεΒΡΜ·―ß ΫΘΚ___ΓΘ
ΔΎ“―÷ΣLaΈΣ+3ΦέΘ§Β±±ΜΗΤΒ»ΕΰΦέ‘ΣΥΊAΧφ¥ζ ±Θ§Ω…–Έ≥…Η¥ΚœΗΤν―ΩσΜ·ΚœΈοLa1-xAxMnO3Θ§Θ®x<0.1Θ©Θ§¥Υ ±“Μ≤ΩΖ÷ΟΧΉΣ±δΈΣ+4ΦέΓΘΒΦ÷¬≤ΡΝœ‘ΎΡ≥“ΜΈ¬Ε»ΗΫΫϋ”–Ζ¥Χζ¥≈ΓΣΧζ¥≈ΓΔΧζ¥≈ΓΣΥ≥¥≈ΉΣ±δΦΑΫπ τΓΣΑκΒΦΧεΒΡΉΣ±δΘ§‘ρLa1-xAxMnO3÷–»ΐΦέΟΧ”κΥΡΦέΟΧΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣΘΚ___ΓΘΘ®”ΟΚ§xΒΡ¥ζ ΐ Ϋ±μ ΨΘ©
ΔέMnΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣΘΚ___ΓΘ
Δήœ¬Ν–”–ΙΊΥΒΖ®’ΐ»ΖΒΡ «___ΓΘ
A.ογΓΔΟΧΓΔ―θΖ÷±πΈΜ”Ύ÷ήΤΎ±μfΓΔdΓΔp«χ
B.―θΒΡΒΎ“ΜΒγάκΡή±»ΒΣΒΡΒΎ“ΜΒγάκΡή¥σ
C.ΟΧΒΡΒγΗΚ–‘ΈΣ1.59Θ§CrΒΡΒγΗΚ–‘ΈΣ1.66Θ§ΥΒΟςΟΧΒΡΫπ τ–‘±»Ηθ«Ω
D.ΗθΒΡΕ―ΜΐΖΫ Ϋ”κΦΊœύΆ§Θ§‘ρΤδΕ―ΜΐΖΫ Ϋ»γΆΦ2
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΖέΡ©Ή¥ ‘―υA «”…Β»Έο÷ ΒΡΝΩΒΡMgOΚΆFe2O3Ήι≥…ΒΡΜλΚœΈοΓΘΫχ––»γœ¬ Β―ιΘΚ
ΔΌ»Γ ΝΩAΫχ––¬Ν»»Ζ¥”ΠΘ§≤ζΈο÷–”–ΒΞ÷ B…ζ≥…ΘΜ
ΔΎΝμ»Γ20 g A»Ϊ≤Ω»ή”Ύ0.15 L 6.0 molΓΛLΘ≠1―ΈΥα÷–Θ§ΒΟ»ή“ΚCΘΜ
ΔέΫΪΔΌ÷–ΒΟΒΫΒΡΒΞ÷ BΚΆ»ή“ΚCΖ¥”ΠΘ§Ζ≈≥ω1.12 L(±ξΩω)ΤχΧεΘ§Ά§ ±…ζ≥…»ή“ΚDΘ§ΜΙ≤–Ντ”–ΙΧΧεΈο÷ BΘΜ
Δή”ΟKSCN»ή“ΚΦλ―ι ±Θ§»ή“ΚD≤Μ±δ…ΪΓΘ
«κΧνΩ’ΘΚ
(1)ΔΌ÷–“ΐΖΔ¬Ν»»Ζ¥”ΠΒΡ Β―ι≤ΌΉς «___________Θ§≤ζΈο÷–ΒΡΒΞ÷ B «________ΓΘ
(2)ΔΎ÷–ΥυΖΔ…ζΒΡΗςΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «___________ΓΘ
(3)Δέ÷–ΥυΖΔ…ζΒΡΗςΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «___________ΓΘ
(4)»τ»ή“ΚDΒΡΧεΜΐ»‘ ”ΈΣ0.15 LΘ§‘ρΗΟ»ή“Κ÷–c(Mg2ΘΪ)ΈΣ________Θ§c(Fe2ΘΪ)ΈΣ________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΤ·ΑΉΖέ‘Ύ…γΜα…ζΜνΓΔΙΛ“Β…ζ≤ζ÷–”ΟΆΨΙψΖΚΘ§Τ·ΑΉΖέ≥ΐΝΥΨΏ”–Τ·ΑΉΉς”ΟΆβΘ§ΜΙΡή…±ΨζœϊΕΨΓΘΚιΥ°ΙΐΚσΘ§‘÷«χ»ΥΟ«ΒΡ“ϊ”ΟΥ°±Ί–κ”ΟΤ·ΑΉΖέΒ»“©ΤΖœϊΕΨΚσ≤≈Ρή“ϊ”ΟΘ§“‘Ζά¥Ϊ»Ψ≤ΓΖΔ…ζΘ§ΒΪΤΩΉΑΤ·ΑΉΖέΨΟ÷ΟΩ’Τχ÷–Μα≥ œΓ÷ύΉ¥Εχ ß–ßΓΘ
(1)Τ·ΑΉΖέΩ…”Οά¥Τ·ΑΉΜρ…±ΨζœϊΕΨΘ§”ΟΜ·―ßΖΫ≥Χ Ϋ±μ ΨΤδ‘≠άμΘΚ________________ΓΘ
(2)”ΟΜ·―ßΖΫ≥Χ Ϋ±μ ΨΤ·ΑΉΖέ‘ΎΩ’Τχ÷–“Ή ß–ßΒΡ‘≠“ρΘΚ________________________ΓΘ
(3)“―÷Σ≈®―ΈΥαΚΆ¥Έ¬»ΥαΗΤΡήΖΔ…ζ»γœ¬Ζ¥”ΠΘΚCa(ClO)2+4HCl(≈®)=CaCl2+2Cl2Γϋ+2H2OΘ§”Ο÷ϋ¥φΚήΨΟΒΡΤ·ΑΉΖέ”κ≈®―ΈΥα÷ΤΒΟΒΡ¬»Τχ÷–Θ§Ω…ΡήΚ§”–ΒΡ‘”÷ ΤχΧε «_______________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΗυΨίΑ±ΤχΜΙ‘≠―θΜ·Ά≠ΒΡΖ¥”ΠΘ§Ω……ηΦΤ≤βΕ®Ά≠‘ΣΥΊœύΕ‘‘≠Ή”÷ ΝΩArΘ®CuΘ© (ΫϋΥΤ÷Β)ΒΡ Β―ιΓΘœ»≥ΤΝΩΖ¥”ΠΈο―θΜ·Ά≠ΒΡ÷ ΝΩm(CuO)Θ§Ζ¥”ΠΆξ»ΪΚσ≤βΕ®…ζ≥…ΈοΥ°ΒΡ÷ ΝΩm(H2O)Θ§”…¥ΥΦΤΥψArΘ®CuΘ©ΓΘΈΣ¥ΥΘ§ΧαΙ©ΒΡ Β―ι“«ΤςΦΑ ‘ΦΝ»γœ¬(ΗυΨί–η“ΣΩ…÷ΊΗ¥―Γ”ΟΘ§Φ”»κΒΡNH4C1”κCa(OH)2ΒΡΝΩΉψ“‘≤ζ…ζ ΙCuOΆξ»ΪΜΙ‘≠ΒΡΑ±Τχ)ΘΚ
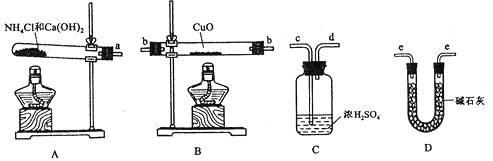
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)Α±ΤχΜΙ‘≠≥ψ»»―θΜ·Ά≠ΒΡΜ·―ßΖΫ≥Χ ΫΈΣ________________________________ΘΜ
(2)¥”ΥυΧαΙ©ΒΡ“«ΤςΦΑ ‘ΦΝ÷–―Γ‘ώ≤ΔΉιΉΑ±Ψ Β―ιΒΡ“ΜΧΉΚœάμΓΔΦρΒΞΒΡΉΑ÷ΟΘ§Α¥ΤχΝςΖΫœρΒΡΝ§Ϋ”Υ≥–ρΈΣ(”ΟΆΦ÷–±ξΉΔΒΡΒΦΙήΩΎΖϊΚ≈±μ Ψ)aΓζ______________________________ΘΜ
(3)‘Ύ±Ψ Β―ι÷–Θ§»τ≤βΒΟm(CuO)=agΘ§m(H2O)=bgΘ§‘ρArΘ®CuΘ©= _______________ΘΜ
(4)‘Ύ±Ψ Β―ι÷–Θ§ Ι≤βΕ®ΫαΙϊArΘ®CuΘ©ΤΪ¥σΒΡ «_______________ (Χν–ρΚ≈)ΘΜ
ΔΌCuOΈ¥Άξ»ΪΤπΖ¥”Π ΔΎ CuO≤ΜΗ…‘ο
ΔέCuO÷–Μλ”–≤ΜΖ¥”ΠΒΡ‘”÷ ΔήΦν ·Μ“≤ΜΗ…‘ο
ΔίNH4C1”κCa(OH)2ΜλΚœΈο≤ΜΗ…‘ο
(5)‘Ύ±Ψ Β―ι÷–Θ§ΜΙΩ…Ά®Ιΐ≤βΕ®_______________ΚΆ_______________Θ§Μρ_______________ΚΆ_______________¥οΒΫ Β―ιΡΩΒΡΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
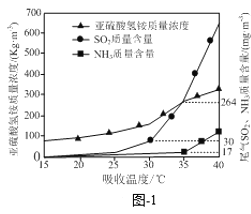
ΓΨΧβΡΩΓΩœ¬Ν–ΆΦ Ψ”κΕ‘”ΠΒΡ–π ω’ΐ»ΖΒΡ «
A.ΆΦΦΉ±μ Ψ“ΜΕ®ΧθΦΰœ¬Ζ¥”Π2SO2(g) + O2(g)![]() 2SO3(g)÷–ΗςΈο÷ ΒΡΈο÷ ΒΡΝΩ≈®Ε»Υφ ±ΦδΒΡ±δΜ·Θ§ΥΒΟςt2 ±ΩΧΫωΥθ–ΓΝΥ»ίΤςΒΡ»ίΜΐ
2SO3(g)÷–ΗςΈο÷ ΒΡΈο÷ ΒΡΝΩ≈®Ε»Υφ ±ΦδΒΡ±δΜ·Θ§ΥΒΟςt2 ±ΩΧΫωΥθ–ΓΝΥ»ίΤςΒΡ»ίΜΐ
B.ΆΦ““±μ ΨΖ¥”ΠCO2(g) + 3H2(g) ![]() CH3OH(g) + H2O(g) ΠΛHΘΦ0‘ΎΚψ»ίΟή±’»ίΤς÷–Θ§ΤδΥϊΧθΦΰœύΆ§ ±Θ§ΫωΗΡ±δΖ¥”ΠΈ¬Ε»Θ§n(CH3OH)Υφ ±ΦδΒΡ±δΜ·Θ§ΥΒΟςKΔώΘΨKΔρ
CH3OH(g) + H2O(g) ΠΛHΘΦ0‘ΎΚψ»ίΟή±’»ίΤς÷–Θ§ΤδΥϊΧθΦΰœύΆ§ ±Θ§ΫωΗΡ±δΖ¥”ΠΈ¬Ε»Θ§n(CH3OH)Υφ ±ΦδΒΡ±δΜ·Θ§ΥΒΟςKΔώΘΨKΔρ
C.ΆΦ±ϊ±μ Ψ”Ο0.01 molΓΛL1 AgNO3»ή“ΚΒΈΕ®≈®Ε»ΨυΈΣ0.01 molΓΛL1ΒΡNaXΓΔNaYΜλΚœ»ή“Κ ±Θ§Θ≠lgcΥφAgNO3»ή“ΚΧεΜΐΒΡ±δΜ·Θ§ΥΒΟςKsp(AgY) ΘΨKsp(AgX)
D.ΆΦΕΓ±μ Ψ25Γφ ±Θ§Φ”Υ°œΓ Ά10 mL pHΨυΈΣ5ΒΡHF”κHCN»ή“Κ ±Θ§»ή“ΚΒΡpHΥφ»ή“ΚΧεΜΐΒΡ±δΜ·Θ§ΥΒΟςKa(HCN) ΘΨKa(HF)
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ”––ßΆ―≥ΐ―ΧΤχ÷–ΒΡSO2 «ΜΖΨ≥±ΘΜΛΒΡ÷Ί“ΣΩΈΧβΓΘ
(1)Α±Υ°Ω…“‘Ά―≥ΐ―ΧΤχ÷–ΒΡSO2ΓΘΑ±Υ°Ά―ΝρΒΡœύΙΊ»»Μ·―ßΖΫ≥Χ Ϋ»γœ¬ΘΚ
2NH3(g) +H2O(l) +SO2(g) =(NH4)2SO3(aq) ΠΛH= akJΓΛmol1
(NH4)2SO3(aq)+H2O(l) +SO2(g) =2NH4HSO3(aq) ΠΛH = bkJΓΛmol1
2(NH4)2SO3(aq) +O2(g) =2(NH4)2SO4(aq) ΠΛH =ckJΓΛmol1
Ζ¥”ΠNH3(g) +NH4HSO3(aq) +![]() O2(g) = (NH4)2SO4(aq)ΒΡΠΛH=____kJΓΛmol1ΓΘ
O2(g) = (NH4)2SO4(aq)ΒΡΠΛH=____kJΓΛmol1ΓΘ
“―÷ΣΘΚSO2ΒΡΙζΦ“≈≈Ζ≈±ξΉΦΈΣ80mgΓΛm3ΓΘΑ±Υ°Ά―≥ΐ―ΧΤχ÷–ΒΡSO2 «‘ΎΈϋ ’Υΰ÷–Ϋχ––ΒΡΘ§ΩΊ÷ΤΤδΥϊ Β―ιΧθΦΰœύΆ§Θ§ΫωΗΡ±δΈϋ ’ΥΰΒΡΈ¬Ε»Θ§ Β―ιΫαΙϊ»γΧβΆΦ-1Υυ ΨΘ§ΈΣΝΥΨΓΩ…ΡήΜώΒΟNH4HSO3Θ§‘ρΈϋ ’ΥΰΚœ ΒΡΈ¬Ε»‘ΦΈΣ________ΓΘ
AΘ°25Γφ BΘ°31Γφ CΘ°35Γφ
(2)ΒγΫβΖ®Ω…“‘Ά―≥ΐ―ΧΤχ÷–ΒΡSO2ΓΘ”ΟNa2SO4»ή“ΚΈϋ ’―ΧΤχ÷–ΒΡSO2Θ§ Ι”ΟΕη–‘ΒγΦΪΒγΫβΈϋ ’ΚσΒΡ»ή“ΚΘ§H2SO3‘Ύ“θΦΪ±ΜΜΙ‘≠ΈΣΝρΒΞ÷ Θ§“θΦΪΒΡΒγΦΪΖ¥”Π ΫΈΣ_______ΓΘ
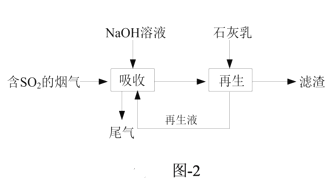
(3)ΡΤΗΤΥΪΦνΖ®Ω…ΗΏ–ßΆ―≥ΐ―ΧΤχ÷–ΒΡSO2Θ§Ά―ΝρΒΡΝς≥Χ»γΧβΆΦ-2Υυ ΨΓΘ
ΔΌΓΑΈϋ ’Γ± ±Τχ“ΚΡφΝς‘ΎΈϋ ’Υΰ÷–Ϋ”¥ΞΘ§Έϋ ’ ±≤Μ“Υ÷±Ϋ” Ι”Ο ·Μ“»ιΒΡ‘≠“ρ «_______ΓΘ

ΔΎΥ°»ή“Κ÷–H2SO3ΓΔHSO![]() ΓΔSO
ΓΔSO![]() ΥφpHΒΡΖ÷≤Φ»γΧβΆΦ3Υυ ΨΘ§ΓΑ‘Ό…ζ“ΚΓ±”ΟNaOH»ή“ΚΒςpH÷Ν7~9ΒΟΒΫ»ή“ΚXΘ§»ή“ΚXΈϋ ’SO2 ±÷ς“ΣΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ______ΓΘ
ΥφpHΒΡΖ÷≤Φ»γΧβΆΦ3Υυ ΨΘ§ΓΑ‘Ό…ζ“ΚΓ±”ΟNaOH»ή“ΚΒςpH÷Ν7~9ΒΟΒΫ»ή“ΚXΘ§»ή“ΚXΈϋ ’SO2 ±÷ς“ΣΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ______ΓΘ
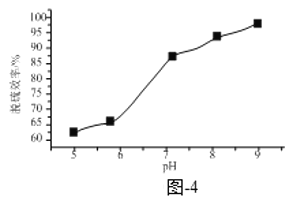
Δέ“―÷ΣNa2SO3ΒΡ»ήΫβΕ»ΥφΉ≈pH‘ω¥σΕχΦθ–ΓΓΘ»ή“ΚXΒΡpHΕ‘Ά―Νρ–߬ ΒΡ”Αœλ»γΧβΆΦ-4Υυ ΨΓΘΒ±pH”…6…ΐΗΏΒΫ7 ±Θ§Ά―Νρ–߬ ―ΗΥΌ‘ω¥σΒΡ‘≠“ρΈΣ______ΘΜΒ±pH¥σ”Ύ7 ±Θ§ΥφpH‘ω¥σΆ―Νρ–߬ ‘ωΥΌΖ≈ΜΚΒΡ‘≠“ρΈΣ______ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–Έο÷ ΒΡ÷Τ±Η”κΙΛ“Β…ζ≤ζœύΖϊΒΡ «
ΔΌNH3![]() NO
NO![]() HNO3
HNO3
ΔΎ≈®HCl![]() Cl2
Cl2 ![]() Τ·ΑΉΖέ
Τ·ΑΉΖέ
ΔέMgCl2(aq)![]() ΈόΥ°MgCl2
ΈόΥ°MgCl2![]() Mg
Mg
Δή±ΞΚΆNaCl(aq)![]() NaHCO3
NaHCO3![]() Na2CO3
Na2CO3
Δί¬ΝΆΝΩσ![]() NaAlO2»ή“Κ
NaAlO2»ή“Κ![]() Al(OH)3
Al(OH)3![]() Al2O3
Al2O3![]() Al
Al
A. ΔΌΔήΔί B. ΔΌΔέΔί C. ΔΎΔέΔή D. ΔΎΔήΔί
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com