
����Ŀ����д���пհ� [�ڣ�1������4��С����Ԫ�ط�����д]��
��1����������ԭ�Ӱ뾶��С��Ԫ��__________��
��2����һ����������Ԫ��__________��
��3���縺������Ԫ��__________��
��4�����������е�һ��������С��Ԫ��__________��
��5������8�����ӣ�10�����ӵ�ԭ�ӵĻ�ѧ����__________��
��6�����������Ų�Ϊ4s24p1��ԭ�ӵĺ˵����Ϊ__________��
��7�����ڱ�������õķǽ���Ԫ��ԭ�ӵĵ����Ų�ͼΪ______________________________��
��8��ijԪ�غ������������Ӳ㣬�����������Ǻ������������1/6��д����Ԫ�ص����ӽṹʾ��ͼ__________��
��9��д����̬ͭԭ�ӵĵ����Ų�ʽ________________��λ��__________����
���𰸡�Cl He F K ![]() 31
31 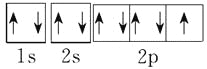
 1s22s22p63s23p63d104s1��[Ar]3d104s1 ds
1s22s22p63s23p63d104s1��[Ar]3d104s1 ds
��������
��1��ͬ������������ԭ�Ӱ뾶��С�����������ԭ�Ӱ뾶��С��Ԫ��Cl��
��2��ԭ�ӽṹ���ȶ���Ԫ����He���ʵ�һ����������Ԫ��He��
��3���ǽ�����Խǿ���縺��Խ����縺������Ԫ����F��
��4��������Խǿ����һ������ԽС������������е�һ��������С��Ԫ��K��
��5������8�����ӣ�10�����ӵ�ԭ�ӵĻ�ѧ����Ϊ![]() ��
��
��6�����������Ų�Ϊ4s24p1��ԭ�ӵĺ˵����Ϊ2+8+18+3��31��
��7�����ڱ�������õķǽ���Ԫ����F����ԭ�ӵĵ����Ų�ͼΪ ��
��
��8��ijԪ�غ������������Ӳ㣬�����������Ǻ������������1/6����������������x����![]() �����x��2�����Ԫ����Mg����Ԫ�ص����ӽṹʾ��ͼΪ
�����x��2�����Ԫ����Mg����Ԫ�ص����ӽṹʾ��ͼΪ ��
��
��9��ͭ��ԭ��������29����̬ͭԭ�ӵĵ����Ų�ʽ1s22s22p63s23p63d104s1��[Ar]3d104s1��λ��ds����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��s�ܼ���p�ܼ��ĵ���������ͼ���Իش����⡣


(1)s����������ͼ��________�Σ�ÿ��s�ܼ���________��ԭ�ӹ����p����������ͼ��________״��ÿ��p�ܼ���________��ԭ�ӹ������������ϵΪ____________(���ͬ������ͬ��)��
(2)Ԫ��X��ԭ�������ĵ����Ų�ʽΪnsnpn��1��ԭ����������ߵ���________���ӣ�Ԫ��X��������____�������⻯��ĵ���ʽ��________��
(3)��Ԫ��Y��ԭ�������ĵ����Ų�ʽΪnsn��1npn��1����ôY��Ԫ�ط���ӦΪ________��ԭ�ӵĵ����Ų�ͼΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ��ȡ0.l molL-1 HA��Һ��0.1 molL-1 NaOH��Һ�������ϣ���Ϻ���Һ����ı仯���ƣ�����û����Һ��pH��9���Իش��������⣺
��1�������Һ��pH��9��ԭ��_____________________�������ӷ���ʽ��ʾ����
��2�������Һ����ˮ�������c(OH��) =______molL-1 ��0.1 molL-1NaOH ��Һ����ˮ�������c(OH��) =______molL-1 ��
��3��0.l molL-1 HA��Һ��0.05 molL-1 NaOH��Һ�������Ϻ�pH��8����
�� ���û��Һ��c(HA)��c(A��)��c(Na+)��c(OH��)��c(H+) Ũ�ȴӴ�С��˳��Ϊ��_________________��
�� c(HA)+ c(A��)��_______ molL-1��c(HA)��c(A��)��_______molL-1��
��4��25��ʱ����֪NH4A��ҺΪ���ԣ���HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ�� pH________7������������������������������
��5����ͬ�¶�����ͬ���ʵ���Ũ�ȵ�������������Һ��pH�ɴ�С��˳��____������ĸ����
A��NH4HCO3 B��NH4HSO4 C��NH4A D��NH4Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Claus��������Ȼ�����������H2S���յ��������Ƚ�ԭ����ͨ�뷴Ӧ¯���������·�Ӧ��2H2S(g) + 3O2(g) ��2SO2(g) + 2H2O(g)����Ӧ��Ļ������ͨ�봮���Ķ���¶����͵�ת�����ڽ��з�Ӧ��2H2S(g) + SO2(g)![]() 3S(l) + 2H2O(g)������˵����ȷ����
3S(l) + 2H2O(g)������˵����ȷ����
A.��Ӧ�����ĵ���H2Sʱ��ת�Ƶ�����֮��Ϊ2:1
B.���ݹ��������жϷ�Ӧ��Ϊ���ȷ�Ӧ
C.ͨ���������ת�����������Ļ�����
D.��Ӧ��������H2S����ռͨ��H2S������![]() ʱ����Ļ��������
ʱ����Ļ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ���ѧ�ҳɹ��ϳ��˼������ӵ���ĸ��ѿ����ӻ�����(CH3NH3)PbI3������������нϸߵĹ��ת��Ч�ʶ���̫���ܵ�����������Ҫ��Ӧ�ü�ֵ���ش���������
��1��C��N��̬ԭ���У���һ�����ܽϴ����_____��
��2��CH3NH3+�ĵ���ʽΪ_____��C��Nԭ�ӵ��ӻ�������ͷֱ�Ϊ______��______��CH3NH3+�У�����______������ţ���
a������ b������ c����� d�����
��֪���Ĺ���������ǿ����ԭ�ӣ���CH3NH2��(CH3)2NH�н�������������ǿ����______��
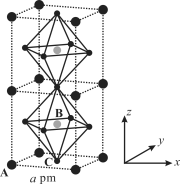
��3��(CH3NH3)PbI3����������ṹ��ͼ��ʾ������B����Pb2+����______���� I��ÿ�������к���I ����ĿΪ______��ԭ�ӷ�����������ڱ�ʾ�����ڲ���ԭ�ӵ����λ�á����У�ԭ�ӷ�������AΪ��0��0��0����BΪ��1/2��1/2��1/2������C��ԭ�ӷ�������Ϊ______����֪(CH3NH3)PbI3�ľ�������Ϊa pm�������ܶ�Ϊ g��cm3����NAΪ�����ӵ�������ֵ����(CH3NH3)PbI3��Ħ������Ϊ________g��mol1���ô���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ�������ʵ�ı�������ȷ���ǣ� ��
A. ��H��Cl�γɹ��ۼ��Ĺ��̣�![]()
B. Na2O2���ں�����ߵĹ�������2Na2O2+2CO2��2Na2CO3+O2
C. ʵ������NH4Cl��Ca(OH)2�Ļ������ȡ����2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O
D. ���Ե缫��ⱥ��ʳ��ˮ�����ӷ���ʽ��2Cl-+2H2O![]() 2OH-+H2��+Cl2��
2OH-+H2��+Cl2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵�����ʾ��������ȷ����
A. ������ȼ����Ϊ285.8 kJ��mol��1��������ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g)��O2(g)=2H2O(l) ��H����285.8 kJ��mol��1
B. ������������������۷ֱ���ȫȼ�գ����߷ų���������
C. ij�ܱ�����ʢ��0.1 mol N2��0.3 mol H2����һ�������³�ַ�Ӧ,ת�Ƶ��ӵ���ĿС��0.6��6.02��1023
D. ��֪�к���Ϊ57.3 kJ��mol��1��������0.5 mol H2SO4��Ũ������Һ�뺬1 mol NaOH����Һ��ϣ��ų�������ҪС��57.3 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�ϳɰ���Ӧ�Ļ�ѧ����ʽΪ3H2(g)+N2(g)![]() 2NH3(g)������˵���ܱ�ʾ�ϳɰ���Ӧ�ﵽ��ѧƽ��״̬����
2NH3(g)������˵���ܱ�ʾ�ϳɰ���Ӧ�ﵽ��ѧƽ��״̬����
A. v��(N2)=3v��( H2)
B. ����H��H �����ѵ�ͬʱ������N��H ������
C. NH3�İٷֺ������ֲ���
D. c(N2) : c(H2) : c(NH3)=1 : 3 : 2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ɫ����A��һ�ֻ���ɫ����B�п�������ȼ�գ�������ɫ���棬��Ӧ��������C��B�����D��Ӧ�����ɰ�ɫ����E��D�ڿ�����ȼ������dz��ɫ����F��D��ˮ��Ӧ�ֿ�����A���Իش��������⣺

��1��д���������ʵĻ�ѧʽ��B________��C_________��E___________��
��2��д��B��ˮ��Ӧ�����ӷ���ʽ��_________________________________
��3��Ϊ����֤Fe3+�����ʣ�ij��ѧ��ȤС���������ͼ��ʾ��һ��ʵ�飬ʵ�鷽����ƴ������____������ĸ��
A�������� B��ֻ���� C�������� D���٢ڢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com