
����Ŀ���������Ƭ�Ķ�Ӱ�����У�δ�ع���廯��(AgBr)�������������(Na2S2O3)�ܽ⣬��Ӧ����Na3[Ag(S2O3)2]���ڷ϶�ӰҺ�м���Na2SʹNa3[Ag(S2O3)2]�е���ת��ΪAg2S����ʹ��ӰҺ��������Ag2S�ڸ�����ת��ΪAg���ʹﵽ�˻�������Ŀ�ġ�
(1)ͭ����������Ԫ�����ڱ���Ϊ���ɽ���Ԫ�أ����л�̬��ԭ�ӵļ۵����Ų�ʽΪ______��
(2)Na��O��S�����Ӱ뾶�ɴ�С��˳��Ϊ___________���ü����ӷ��ű�ʾ���Ӱ뾶����
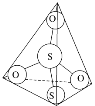
(3)S2O32-���ӽṹ��ͼ1��ʾ����������ԭ�ӵ��ӻ��������Ϊ________��
(4)Na3[Ag(S2O3)2]�д��ڵ���������_____________��
A�����Ӽ� B�����ۼ� C�����»��� D�������� E.��λ��
(5)�ڿ���������Ag2S����Ag��SO2��SO2���ӿռ乹��Ϊ____________________����SO3��ȣ�____________�ļ��Ǹ���ԭ����________________________________��

(6)��ͼ�ǽ����⻯�ﴢ����ϣ��侧����ͼ��ʾ���仯ѧʽΪ____________________����֪�þ�����ܶ�Ϊ��g/cm3����þ��������Ϊ_________ cm3���ú��ѡ�NA�Ĵ���ʽ��ʾ����
���𰸡�3d54s1 S2����O2����Na�� sp3 ABE V�� SO3 SO2��SO3����ԭ�Ӿ�Ϊsp2�ӻ���SO2����һ�Թµ��Ӷԣ����������ų����ø��� MgH2 ![]()
��������
��1����̬ͭԭ�Ӻ�����29�����ӣ���3d��4s�ܼ��ϵĵ���Ϊ��۵��ӣ����ݹ���ԭ����д��̬Cuԭ�ӵļ۵����Ų�ʽ��
��2�����Ӻ�����Ӳ���Խ�࣬�����Ӱ뾶Խ���Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С��
��3��S2O32-���ӽṹ��������ԭ�ӵļ۲���ӶԸ�����4�����ݼ۲���ӶԻ��������ж�����Sԭ�ӵ��ӻ�������ͣ�
��4��Na3[Ag��S2O3��2]����������֮��������Ӽ���Ag���Ӻ����������λ����S-Oԭ��֮����ڼ��Թ��ۼ���
��5�����ݼ۲���ӶԻ��������жϸ÷��ӿռ乹�ͣ�SO3�м۵��Ӷ�Ϊ3��û�й¶Ե��ӣ���SO2�м۵�����Ϊ3���¶Ե�����Ϊ1��
��6�����þ�̯����ѧʽ������![]() �����������⣻
�����������⣻
����1����̬ͭԭ�Ӻ�����29�����ӣ���3d��4s�ܼ��ϵĵ���Ϊ��۵��ӣ����ݹ���ԭ����д��̬Cuԭ�ӵļ۵����Ų�ʽΪ3d104s1��
�ʴ�Ϊ��3d104s1��
��2�����Ӻ�����Ӳ���Խ�࣬�����Ӱ뾶Խ���Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С�����Ӻ�����Ӳ���S2-��࣬O2-��Na+������Ӳ�����ͬ��ԭ������O��Na�������Ӱ뾶�Ӵ�С˳����S2-��O2-��Na+��
�ʴ�Ϊ��S2-��O2-��Na+��
��3��S2O32-���ӽṹ��������ԭ�ӵļ۲���ӶԸ�����4�����ݼ۲���ӶԻ��������ж�����Sԭ�ӵ��ӻ��������Ϊsp3��
�ʴ�Ϊ��sp3��
��4��Na3[Ag��S2O3��2]����������֮��������Ӽ���Ag���Ӻ����������λ����S-Oԭ��֮����ڼ��Թ��ۼ������Ըýṹ�д������Ӽ�����λ�����ۼ���
��ѡABE��
��5��SO2��������ԭ�ӵļ۲���Ӷ���=![]() =3�Һ���1���µ��Ӷԣ����ݼ۲���ӶԻ��������жϸ÷��ӿռ乹��ΪV�Σ�SO3�м۵��Ӷ�Ϊ3��û�й¶Ե��ӣ���SO2�м۵�����Ϊ3���¶Ե�����Ϊ1������SO3�ļ��Ǹ���
=3�Һ���1���µ��Ӷԣ����ݼ۲���ӶԻ��������жϸ÷��ӿռ乹��ΪV�Σ�SO3�м۵��Ӷ�Ϊ3��û�й¶Ե��ӣ���SO2�м۵�����Ϊ3���¶Ե�����Ϊ1������SO3�ļ��Ǹ���
�ʴ�Ϊ��V�Σ�SO3��SO2��SO3����ԭ�Ӿ�Ϊsp2�ӻ���SO2����һ�Թµ��Ӷԣ����������ų����ø���
��6�����ڸþ�������ԭ����ĿΪ2+4��1/2=4�����ڸþ�����þԭ����ĿΪ��8��1/8+1=2�����Է���ʽΪMgH2��![]() ���ʴ�Ϊ��MgH2��
���ʴ�Ϊ��MgH2��![]() ��
��


 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ɫ����X������Ԫ����ɣ�Ϊ̽������ɺ����ʣ����������ʵ�飺

��ش�
(1)����Y��һ��ͬ��������ķ���ʽ��______�����ɫ�����Ļ�ѧʽ______��
(2)X�ڸ��������������ܸ��·ֽ�ΪY��Z�Ļ�ѧ����ʽ____________��
(3)ȡ��ɫ��ҺW�μ��ڵ���KI��ֽ�ϣ���ֽ����ɫ�������ӷ���ʽ��ʾ��ֽ������ԭ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪:��2H2(g)+O2(g)![]() 2H2O(l)����H=-571.6 kJ��mol-1
2H2O(l)����H=-571.6 kJ��mol-1
��2CH3OH(l)+3O2(g)![]() 2CO2(g)+4H2O(l)����H=-1 452 kJ��mol-1
2CO2(g)+4H2O(l)����H=-1 452 kJ��mol-1
��H+(aq)+OH-(aq)![]() H2O(l)����H=-57.3 kJ��mol-1
H2O(l)����H=-57.3 kJ��mol-1
����˵����ȷ����
A. H2(g)��ȼ����Ϊ142.9 kJ��mol-1
B. ͬ������H2(g)��CH3OH(l)��ȫȼ��,H2(g)�ų���������
C. 1/2H2SO4(aq)+1/2Ba(OH)2(aq)![]() 1/2BaSO4(s)+H2O(l)����H=-57.3 kJ��mol-1
1/2BaSO4(s)+H2O(l)����H=-57.3 kJ��mol-1
D. 3H2(g)+CO2(g)![]() CH3OH(l)+H2O(l)����H=+131.4 kJ��mol-1
CH3OH(l)+H2O(l)����H=+131.4 kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W��Ϊ������Ԫ�أ�������Ԫ�����ڱ��е����λ����ͼ��ʾ����Zԭ�ӵ������������ǵ�һ���������3��������˵������ȷ����( )

A. X�������̬�⻯���ˮ��Һ������
B. ����������Ӧˮ���������W��Zǿ
C. Z�ĵ�����������Ӧ��Y������������Ӧ����
D. X��ԭ�Ӱ뾶С��Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о���������ķ�Ӧ����������������Ի�������Ⱦ����Ҫ���塣��ش������뵪Ԫ���йص�����:
(1)��������(�ṹʽΪCl-N=O)���л��ϳ��е���Ҫ�Լ���������Cl2��NO��ͨ�������·�Ӧ�Ƶã���Ӧ����ʽΪ2NO(g)+Cl2(g) ![]() 2ClNO(g)����֪���ֻ�ѧ�ļ����������±���ʾ:
2ClNO(g)����֪���ֻ�ѧ�ļ����������±���ʾ:

��Cl2��NO��Ӧ����ClNO�Ĺ�����ת����4mol���ӣ������Ϸų�������Ϊ____kJ.
(2)��һ�������ܱ������г���2molNO(g)��1 mol Cl2(g)����(1)�з�Ӧ�����¶ȷֱ�ΪT1��T2ʱ���NO�����ʵ���(��λ:mol)��ʱ��Ĺ�ϵ���±���ʾ
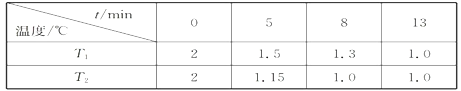
��T1________T2(�>������������)��
���¶�ΪT2��ʱ������ͬ�����У�����4molNO(g)��2mo1Cl2(g)����NO��ƽ��ת����___________50%(����ڡ��������ڡ���С�ڡ�)
���¶�ΪT2��ʱ����ʼʱ�����ڵ�ǿΪp0����÷�Ӧ��ƽ�ⳣ��Kp=______(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
(3)������������ˮ�еĵ���Ⱦ�ѳ�Ϊһ�������ԵĻ������⡣�ڽ���Pt��Cu��ҿ(Ir)�Ĵ������£��ܱ������е�H2�ɸ�Чת��������Һ�е���̬��(NO3-)���乤��ԭ����ͼ��ʾ
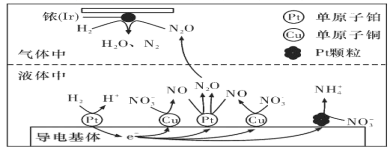
��Ir���淢����Ӧ�ķ���ʽΪ_________________________________________________��
������������ϵ�Pt�������࣬��ɵĺ����___________________________________��
II:���õ绯ѧԭ������NO2��O2������KNO3�Ƴ�ȼ�ϵ�أ�ģ�ҵ��ⷨ����������װ����ͼ��ʾ
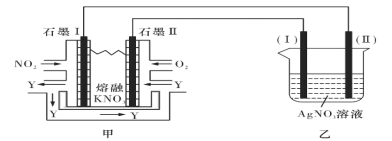
��ش���������:
(4)�ټ׳ع���ʱ��NO2ת�����ɫ������Y��Y��N2O5����ѭ��ʹ�ã���ʯīII���������ĵ缫��ӦʽΪ____________________________________________��
������10A�ĵ������60min����֪�õ��صĵ��Ч��Ϊ80.4���������������õ�____g������Ag��(����С�����һλ��ͨ��һ������ʱ������ʵ�ʳ����Ľ���������ͨ����ͬ����ʱ������Ӧ�����Ľ�������֮�Ƚе��Ч�ʡ������ڳ���Ϊ96500C/mol)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���У��д������( )
A.Na2CO3��Һ����ʯ��ˮ��Ӧ����NaHCO3��Һ������ʯ��ˮ��Ӧ
B.����ͬ�¶��£�NaHCO3���ܽ�ȱ�Na2CO3С
C.Na2CO3���ȶ�����NaHCO3����ʱ���ֽ�
D.�������ʵ�����NaHCO3��ĩ��Na2CO3��ĩͬʱ�ֱ�����������ͬŨ�ȵ�ϡ�����У�ǰ�ߵķ�Ӧ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����2H2O(g)===2H2(g)��O2(g)����H����483.6 kJ��mol��1 ��H2S(g)===H2(g)��S(g)����H����20.1 kJ��mol��1�����ж���ȷ����(����)
A. ������ȼ���ȣ���H����241.8 kJ��mol��1
B. ��ͬ�����£����ȼ��1 mol H2(g)��1 mol S(g)�Ļ����ȳ��ȼ��1 mol H2S(g)���ȶ�20.1 kJ
C. �ɢ٢�֪��ˮ�����ȶ���С������
D. ���������ɹ�̬����H������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ɱ��400 ���ܱ������У�һ������SO2��O2�ڴ��������·�����Ӧ��2SO2(g)+O2(g) 2SO3(g)+QkJ����������������ǣ� ��
2SO3(g)+QkJ����������������ǣ� ��
A.����ѹǿ������Ӧ����һֱ���������䣬ƽ������
B.�����¶ȣ�����Ӧ���ʱ��淴Ӧ���ʼ�С�ij̶�С
C.��������������������ƽ�������ƶ���ƽ�ⳣ��Kֵ����
D.����������ʵ�������0.5molʱ�ﵽƽ�⣬��������·�Ӧ�ų�0.5QkJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����10 L�ܱ������У�1 mol A��3 mol B��һ�������·�Ӧ�� A(g)��xB(g)![]() 2C(g)��2 min��Ӧ�ﵽƽ��ʱ����û�����干3.4 mol������0.4 mol C�������м�������ȷ����
2C(g)��2 min��Ӧ�ﵽƽ��ʱ����û�����干3.4 mol������0.4 mol C�������м�������ȷ����
A. ƽ��ʱ�����ʵ���֮��n(A)��n(B)��n(C)��2��11��4
B. xֵ����3
C. A��ת����Ϊ20%
D. B��ƽ����Ӧ����Ϊ0.4 mol/(L��min)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com