
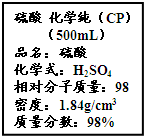
 ��Ҫ���������С�⣮
��Ҫ���������С�⣮| m |
| n |
| 1000w |
| M |
| 1.08g |
| 0.01mol |
| 1000��1.84��98% |
| 98 |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������п�̵�� | B��Ǧ���� |
| C�����ӵ�� | D������ӵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 2 |
| 3 |
| A��ZO3��ˮ��Ӧ�γɵĻ����������ӻ����� |
| B��ճ���Թ��ڱ��ϵ�Z������YZ2ϴ�� |
| C������������Ӧˮ����ļ��ԣ�X��W |
| D��Xλ�ڽ�����ǽ����ķֽ��ߴ������������뵼����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
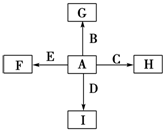
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1������ʽΪC4H10O�Ĵ�����
��1������ʽΪC4H10O�Ĵ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C����Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������B2-�Ľṹʾ��ͼΪ
A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C����Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������B2-�Ľṹʾ��ͼΪ �����û�ѧ����ش��������⣺
�����û�ѧ����ش��������⣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com