
����Ŀ��(1)ij�о�С�齫V1 mL 1.0 mol/L HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ��ʾ(ʵ����ʼ�ձ���V1��V2��50 mL)������ɼ�ͼ�ο�֪��V1��V2��________ʱ�����ǡ����ȫ�кͣ��˷�Ӧ����NaOH��Һ��Ũ��ӦΪ__________mol/L����NaOH��Һ����ͬŨ�Ⱥ������������Һ���棬����к�����ֵ�ⶨ��������Ӱ��(����ƫ��������ƫС��������Ӱ����)��KOH��Һ______����ˮ(NH3��H2O)___________��

(2)�ö��Ե缫���е�����е������Һ��
�ٵ���Ȼ�ͭ��Һ,�������Ϻ�������������������ʵ���֮��Ϊ___________
��MnO2�����������������ϣ����MnSO4��Һ���Ƶ�MnO2���������ĵ缫��Ӧʽ_________________
(3)�����£�0.1mol/L��������(HNO2)��������ĵ��볣��Ka�ֱ�Ϊ�� 7.1 10-4�� 2.98 10-8����0.1mol/L��������ϡ��100����c(H+)��_______(��������������������������С��)��д��HNO2��HClO��NaNO2��NaClO��������֮�䷢���ĸ��ֽⷴӦ�����ӷ���ʽ_______________��
(4)��HX�ͼ�AOHǡ����ȫ�к�ʱ��Һ��pH����7����HY�ͼ�BOHǡ����ȫ�к�ʱ��Һ��pHҲ����7����HX�ͼ�BOHǡ����ȫ�к�ʱ��Һ��pHС��7�����ƶ�
����������б�Ϊ������ʵ���_______________
�ڱȽ�������HX��HY������ǿ�� ___________>___________
(5)��ͼΪ������ܵ�صĽṹʾ��ͼ���õ�صĹ����¶�Ϊ320 �����ң���ط�ӦΪ2Na+xS![]() Na2Sx�������ĵ缫��ӦʽΪ_________����Ǧ������ȣ���������ͬ�����ĸ�����������ʱ�������ص����۷ŵ�����Ǧ���ص�__________
Na2Sx�������ĵ缫��ӦʽΪ_________����Ǧ������ȣ���������ͬ�����ĸ�����������ʱ�������ص����۷ŵ�����Ǧ���ص�__________
����

(6)��֪������Fe(OH)3��Mg(OH)2��Ksp�ֱ�Ϊ8.0![]() 10��38��1.0
10��38��1.0![]() 10��11����Ũ�Ⱦ�Ϊ0.1 mol/L��FeCl3��MgCl2�Ļ����Һ�м����Һ��ҪʹFe3����ȫ������Mg2����������Ӧ�õ�����ҺpH�ķ�Χ��________��(��֪lg 2��0.3)
10��11����Ũ�Ⱦ�Ϊ0.1 mol/L��FeCl3��MgCl2�Ļ����Һ�м����Һ��ҪʹFe3����ȫ������Mg2����������Ӧ�õ�����ҺpH�ķ�Χ��________��(��֪lg 2��0.3)
���𰸡�3 �� 2 1.5 ��Ӱ�� ƫС 1 �� 1 Mn2++2H2O-2e-=MnO2+4H+ ��С HNO2+ClO2-=NO2- + HClO BOH��HY HX HY xS+2e-![]() Sx2�� 4.5 3.3��pH<9
Sx2�� 4.5 3.3��pH<9
��������
��1����ͼ���֪ǡ����ȫ��Ӧʱ���¶���ߣ����n(NaOH)=n(HCl)������NaOH��KOH����ǿ���һˮ�ϰ�Ϊ����������ȣ�
��2���ٵ���Ȼ�ͭ��Һ����������������������������ͭ�����ݵ����غ�����������ʵ���֮�ȣ�
����������������Ӧ��Mn2+![]() MnO2���������ҺΪ���Ի��������ݵ����غ���ԭ���غ����д���缫��Ӧʽ��
MnO2���������ҺΪ���Ի��������ݵ����غ���ԭ���غ����д���缫��Ӧʽ��
��3��������Ϊ������ʣ�������ϡ�ͣ�����̶���Ȼ��������ϡ�͵�����Һ��������Ũ�Ƚ��ͣ�����ͬŨ�������ᡢ��������볣����֪�����������Աȴ�����ǿ������ǿ���Ʊ������ԭ����д���ӷ���ʽ��
��4������������������HX�ͼ�AOHǡ����ȫ�к�ʱ��Һ��pH����7������֪����Һ��AX�����ԣ���HY�ͼ�BOHǡ����ȫ�к�ʱ��Һ��pHҲ����7������Һ��BY����������HX�ͼ�BOHǡ����ȫ�к�ʱ��Һ��pHС��7�����Ƴ�B+һ����ˮ�⣬ʹ����Һ��BX�����ԣ���BY����������Y-ˮ���Լ��ԣ���B+ˮ��̶��൱���ݴ˷���������
��5���ɷ���ʽ��֪S���ϼ۽��ͣ�����ԭ��Ϊԭ���������Ӧ���缫����ʽΪxS+2e��Sx2��ԭ��ع���ʱ����������ͬ�����Ľ�������Ǧʱ������ת�Ƶ��ӵ����ʵ����Ĺ�ϵ�ó����ۣ�
��6�������ܶȻ���Ӧ�ã���֪ҪʹFe3����ȫ������Mg2���������������������ӵ�Ũ�ȷ�ΧΪ��![]() ��c��OH-��<
��c��OH-��<![]() ���ٽ��Kw������pHֵ��Χ��
���ٽ��Kw������pHֵ��Χ��
��1����ͼ���֪V1 = 30 mLʱ�¶������˵�����ǡ����ȫ��Ӧ��V1+V2 = 50 mL����V2 = 20 mL��V1��V2 = 3��2������0.03 L��1 mol/L=0.02 L��c��c = 1.5 mol/L��NaOH��KOH����ǿ�����KOH��ʵ����Ӱ�죬��һˮ�ϰ�Ϊ������ʣ��������ȣ����²ⶨ���ƫС��
�ʴ�Ϊ��3��2��1.5����Ӱ�죻ƫС��
��2���ٵ���Ȼ�ͭ��Һ��������������������缫��ӦʽΪ��2Cl��-2e- = Cl2������������ͭ����缫��ӦʽΪ��Cu2++2e- = Cu�������������Ϻ�������������������ʵ���֮��Ϊ1��1��
�ʴ�Ϊ��1��1��
����������������Ӧ��Mn2+![]() MnO2���������ҺΪ���Ի������ʵ缫��ӦʽΪ��Mn2++2H2O-2e-=MnO2+4H+��
MnO2���������ҺΪ���Ի������ʵ缫��ӦʽΪ��Mn2++2H2O-2e-=MnO2+4H+��
�ʴ�Ϊ��Mn2++2H2O-2e-=MnO2+4H+��
��3��������Ϊ���������������ϡ��������̶���Ȼ����������Һ��������Ũ�Ƚ���������ͬŨ�������ᡢ��������볣����֪�����������Աȴ�����ǿ�������֮�䷢�������ӻ�����Ӧ�����ӷ���ʽΪ��HNO2+ClO=NO2+HClO��
�ʴ�Ϊ����С��HNO2+ClO2-=NO2- + HClO��
��4������������������HX�ͼ�AOHǡ����ȫ�к�ʱ��Һ��pH����7������֪����Һ��AX�����ԣ���HY�ͼ�BOHǡ����ȫ�к�ʱ��Һ��pHҲ����7������Һ��BY����������HX�ͼ�BOHǡ����ȫ�к�ʱ��Һ��pHС��7�����Ƴ�B+һ����ˮ�⣬ʹ����Һ��BX�����ԣ���BY����������Y-ˮ���Լ��ԣ���B+ˮ��̶��൱��
������˭��˭ˮ�������ɿ�֪��BOH��HYΪ���������
�ʴ�Ϊ��BOH��HY��
����HXΪǿ�ᣬ�����ԣ�HX HY����HXΪ���ᣬ������HX�ͼ�BOHǡ����ȫ�к�ʱ��Һ��pHС��7�����֪B+ˮ��̶ȴ���X-�ĵ���̶ȣ������Ƴ�Y-ˮ����B+ˮ��̶��൱����Y-��ˮ��̶ȴ���X-�ĵ���̶ȣ��ʸ�����Խ��Խˮ����ԭ����֪��HX�����Դ���HY��������
�ʴ�ΪHX��HY��
��5����������õ��ӷ�����ԭ��Ӧ�����������缫��ӦʽΪ��xS+2e-�TSx2-���Ƹ��ܵ���и���Ϊ�ƣ���23g�������ͷ�1mol e-����207g������ʱת�Ƶ����ʵ��� = ![]() = 9 mol����Ǧ���صĵ缫��ӦΪ��Pb+PbO2+2H2SO4�T2PbSO4+2H2O��Ǧ������Ǧ�Ǹ�����207gǦ����ʱת��2mol e-���������ص����۷ŵ�����Ǧ���ص�9/2 =4.5����
= 9 mol����Ǧ���صĵ缫��ӦΪ��Pb+PbO2+2H2SO4�T2PbSO4+2H2O��Ǧ������Ǧ�Ǹ�����207gǦ����ʱת��2mol e-���������ص����۷ŵ�����Ǧ���ص�9/2 =4.5����
�ʴ�Ϊ��xS+2e-![]() Sx2����4.5��
Sx2����4.5��
��6�������ܶȻ���Ӧ�ã���֪ҪʹFe3����ȫ������Mg2���������������������ӵ�Ũ�ȷ�ΧΪ ��![]() ��c��OH-��<
��c��OH-��<![]() ����
����![]() mol/L��c��OH-��<
mol/L��c��OH-��<![]() mol/L��2
mol/L��2![]() 10-11 mol/L��c��OH-��< 10-5 mol/L������KW=c(H+)��c(OH)��֪��c(H+)��ȡֵ��ΧΪ��
10-11 mol/L��c��OH-��< 10-5 mol/L������KW=c(H+)��c(OH)��֪��c(H+)��ȡֵ��ΧΪ��![]() mol/L< c(H+) ��
mol/L< c(H+) �� ![]() mol/L��pH = -lg c(H+)����pHֵ��ΧΪ3.3 �� pH< 9��
mol/L��pH = -lg c(H+)����pHֵ��ΧΪ3.3 �� pH< 9��
�ʴ�Ϊ3.3 �� pH< 9��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ����٤��������ֵ��������˵����ȷ����
A. ��״���£�2.24L N2��O2�Ļ�������з�����Ϊ0.1NA
B. 32g S8�����ӽṹ��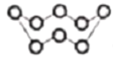 ���еĹ��ۼ���ĿΪ8NA
���еĹ��ۼ���ĿΪ8NA
C. 3mol SO2��1mol O2���ܱ������д���Ӧ���������Ϊ3NA
D. pH=1��H3BO3��Һ�У�����H+������Ϊ0.3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڷ�Ӧ�ȵı�����ȷ����
A. ����HΪ������ʱ����ʾ�÷�ӦΪ���ȷ�Ӧ
B. ��֪C(s)+![]() O2(g)====CO(g)�ķ�Ӧ��Ϊ110.5 kJ/ mol��˵��̼��ȼ����Ϊ110.5 kJ / mol
O2(g)====CO(g)�ķ�Ӧ��Ϊ110.5 kJ/ mol��˵��̼��ȼ����Ϊ110.5 kJ / mol
C. ��Ӧ�ȵĴ�С�뷴Ӧ�������е������������������е�������
D. 1 mol NaOH�ֱ��1 mol CH3COOH��1 mol HNO3��Ӧ���ų���������CH3COOH��HNO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���������İ���������������Ƶ��ܱ����������(��������������䣬��������������Բ���)���ں㶨�¶���ʹ��ﵽ�ֽ�ƽ�⣺NH2COONH4(s)![]() 2NH3(g)��CO2(g)�������жϸ÷ֽⷴӦ�Ѿ��ﵽ��ѧƽ�����
2NH3(g)��CO2(g)�������жϸ÷ֽⷴӦ�Ѿ��ﵽ��ѧƽ�����
A. �ܱ������ж�����̼������������� B. �ܱ�����������������ʵ����ı�
C. �ܱ������л��������ܶȲ��� D. 2v��(NH3)��v��(CO2)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij��ɫ����Һ�н����а���Na+��CH3COO�����ڵ��������ӡ�
��ش��������⡣
��1������Һ�е����ʿ����������������ֱ�д����������Ļ�ѧʽ��
��_____________________����________________________����____________________ ��
��2��25�棬����0.1 mol/L��CH3COOH��Һ��0.1 mol/L��NaOH��Һ�������ϣ���û����Һ��pH=8����c(CH3COOH)+c(H+)�ľ�ȷֵΪ___________________mol/L��
��3��������Һ��pH=8����c(Na+)-c(CH3COO��)�ľ�ȷֵΪ___________________mol/L��
��4������Һ�и�����Ũ�ȴ�С��˳��Ϊc(CH3COO��)��c(Na+)��c(H+)��c(OH��)ʱ�������Һ������____������ţ���
A����0.1 mol/L��CH3COONa��Һ��0.1 mol/L��CH3COOH��Һ�������϶���
B����0.1 mol/L��CH3COOH��Һ��0.1 mol/L��NaOH��Һ�������϶���
C����0.2 mol/L��CH3COONa��Һ��0.1 mol/L��HCl��Һ�������϶���
D����0.2 mol/L��CH3COOH��Һ��0.1 mol/L��NaOH��Һ�������϶���
��5�������£���a mol/L��CH3COOH��Һ��0.01 mol/L��NaOH��Һ�������ϣ���Ӧƽ�����Һ��c(Na+)=c(CH3COO��)���ú�a�Ĵ���ʽ��ʾCH3COOH�ĵ���ƽ�ⳣ��Ka=_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͪ��ң�H����һ��������ʹҩ����ϳ�·�����£�

��֪R-CN![]() R-COOH
R-COOH
�ش��������⣺
��1��A�Ļ�ѧ������____��
��2����B����C�Ļ�ѧ����ʽΪ________��
��3��D�Ľṹ��ʽΪ_______��
��4��F�й����ŵ�����Ϊ_____��
��5��G����H���Լ�X�ķ���ʽΪC6H8N2���ķ�Ӧ������____��
��6��E�ķ���ʽΪ____��X��E��Ϊͬ���칹�壬X�ķ����к�����������Xˮ������֮һ���ܷ���������Ӧ������FeCl3��Һ������ɫ��Ӧ��X�ĺ˴Ź���������ʾ��6�ֲ�ͬ��ѧ�������⣬�����֮��Ϊ1��2��2��2��2��3��д������������X�Ľṹ��ʽ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ�������������( )

A. ͼ�ױ�ʾ��CH3COOH��Һ������CH3COONa�������ҺpH�ı仯
B. ͼ�ұ�ʾ��CH3COOH��Һ�м�ˮʱ��Һ�ĵ����Ա仯����CH3COOH��Һ��pH��a>b
C. ͼ����ʾ�����ܸı仯ѧ��Ӧ���ʱ�
D. ͼ����ʾ����NO2���ݻ���ͬ�ĺ����ܱ������У���ͬ�¶��·ֱ�����Ӧ��2NO2(g)![]() N2O4(g)����ͬʱ�����NO2���������ߣ���÷�Ӧ����H<0
N2O4(g)����ͬʱ�����NO2���������ߣ���÷�Ӧ����H<0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1������KCl��Һ��K2CO3���Լ���____________�����ӷ���ʽΪ____________________��
��2����ȥNa2CO3��ĩ�л����NaHCO3������______________________��������ѧ����ʽΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2O2����Ҫ�Ļ���ԭ�ϣ����ж�����;��
��1��Na2O2���������ԣ����Խ�SO2����Ϊ�����ƣ�д���÷�Ӧ�Ļ�ѧ����ʽ��______________________���÷�Ӧ�У�Na2O2������Ϊ____________��������ԭ���������������������������������ǻ�ԭ��������
��2��Na2O2��CO2��Ӧ���Բ���������ijͬѧͨ������װ����֤Na2O2�ܷ���CO2��Ӧ�� (ͼ������̨��װ������ȥ)��

��װ��A��������_________��A�еĹ���Ϊ_______________��װ��B���Լ�������Ϊ__________________________
����Na2O2����CO2����װ��C�е�������_____________________________
��3����ʯ���Ǹ��������a���ռ����壬��ⷢ�ָ������м�������CO2���壨������������������˵������������CO2���岻��Ӧ����ͬѧ����������ף�Ȼ��װ��B�������������������Լ��������Ӻ�װ�ú��ٴν���ʵ�飬�����ռ������⣬���ֵõ������弸��������������ʵ����˵������������CO2���巴Ӧ��Ҫ_______________��
��4����һ������Na2O2����Ͷ�뵽�����������ӵ���Һ�У�NO3����HCO3-��CO32����Na������Ӧ��Ϻ���Һ������������Ŀ����������У���������Һ����ı仯��__________�������ӷ��ţ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com