
����Ŀ���߾���H�����ڹ�̹����У�������ʴͿ�㡣�����Ǹ߾���H�ĺϳ�·�ߣ�
![]()

��.��Ӧ�����ڼӾ۷�Ӧ ��.D���ڸ߷��ӻ����
��ش��������⣺
(1)��Ӧ�ݵ�������_______________��E�ķ���ʽΪ__________________��
(2)C�к��еĹ����ŵ�������________________ ��
(3)������M��A��ʵ��ʽ��ͬ�����ⶨM�ĺ˴Ź���������5����ҷ������Ϊ1:1:2:2:2,��M�Ľṹ��ʽΪ_____________ ���Ʋ�M���ܷ����ķ�Ӧ������___________��(д��һ�ּ���)
(4)��Ӧ�ߵĻ�ѧ����ʽΪ___________________________________________________ ��
(5)D��G��Ӧ����H�Ļ�ѧ����ʽΪ___________________________________________ ��
(6)G��ͬ���칹���У���G������ͬ�����ŵķ����廯���ﻹ��__________��(�����������칹)��
���𰸡����� C7H8O ���� ![]() �ӳɷ�Ӧ��Ӿ۷�Ӧ��������Ӧ
�ӳɷ�Ӧ��Ӿ۷�Ӧ��������Ӧ ![]()

 4
4
��������
̼������ˮ��Ӧ������Ȳ��A��������C�Ľṹ��ʽ��֪B�Ľṹ��ʽΪCH2=CH-OOCCH3��������Ȳ��������һ�������·����ӳɷ�Ӧ����CH2=CH-OOCCH3���л���C�ڼ��������·���ˮ�����ɸ߷��ӻ�����D�� ���л��� XΪ�ױ������������������ϵ�ȡ����Ӧ������Y��C6H5-CH2Cl��Y�ڼ���������ˮ��������E��C6H5-CH2OH���л���E������Ϊ(F )C6H5-CHO��������Ϣ��֪��C6H5-CHO��CH3COOH��һ�������·�����Ӧ������G��C6H5-CH=CH2COOH���߷��ӻ�����D��G�����������ɸ߷��ӻ�����H�������Ϸ��������
���л��� XΪ�ױ������������������ϵ�ȡ����Ӧ������Y��C6H5-CH2Cl��Y�ڼ���������ˮ��������E��C6H5-CH2OH���л���E������Ϊ(F )C6H5-CHO��������Ϣ��֪��C6H5-CHO��CH3COOH��һ�������·�����Ӧ������G��C6H5-CH=CH2COOH���߷��ӻ�����D��G�����������ɸ߷��ӻ�����H�������Ϸ��������
̼������ˮ��Ӧ������Ȳ��A��������C�Ľṹ��ʽ��֪B�Ľṹ��ʽΪCH2=CH-OOCCH3��������Ȳ��������һ�������·����ӳɷ�Ӧ����CH2=CH-OOCCH3���л���C�ڼ��������·���ˮ�����ɸ߷��ӻ�����D�� ���л��� XΪ�ױ������������������ϵ�ȡ����Ӧ������Y��C6H5-CH2Cl��Y�ڼ���������ˮ��������E��C6H5-CH2OH���л���E������Ϊ(F )C6H5-CHO��������Ϣ��֪��C6H5-CHO��CH3COOH��һ�������·�����Ӧ������G��C6H5-CH=CH2COOH���߷��ӻ�����D��G�����������ɸ߷��ӻ�����H��
���л��� XΪ�ױ������������������ϵ�ȡ����Ӧ������Y��C6H5-CH2Cl��Y�ڼ���������ˮ��������E��C6H5-CH2OH���л���E������Ϊ(F )C6H5-CHO��������Ϣ��֪��C6H5-CHO��CH3COOH��һ�������·�����Ӧ������G��C6H5-CH=CH2COOH���߷��ӻ�����D��G�����������ɸ߷��ӻ�����H��
(1)������Ϸ�����֪�� XΪ�ױ������������������ϵ�ȡ����Ӧ������Y��C6H5-CH2Cl����Ӧ����Ϊ���գ�E�Ľṹ��ʽΪC6H5-CH2OH��E�ķ���ʽΪC7H8O������������������������գ�C7H8O��
(2) ����������C�Ľṹ��ʽ��֪���еĹ��������������������������������������
(3) AΪ��Ȳ��ʵ��ʽΪCH��I�����DZ�������ϩ�ȣ�����ΪI�ĺ˴Ź�����������5����ҷ����֮��Ϊ1��1��2��2��2����MΪ����ϩ���ṹ��ʽΪ![]() �������к���̼̼˫�������Է����ӳɷ�Ӧ���Ӿ۷�Ӧ��������Ӧ�����б��������ܹ�����ȡ����Ӧ��M���ܷ����ķ�Ӧ�����Ǽӳɷ�Ӧ��Ӿ۷�Ӧ��������Ӧ������������������ǣ�
�������к���̼̼˫�������Է����ӳɷ�Ӧ���Ӿ۷�Ӧ��������Ӧ�����б��������ܹ�����ȡ����Ӧ��M���ܷ����ķ�Ӧ�����Ǽӳɷ�Ӧ��Ӿ۷�Ӧ��������Ӧ������������������ǣ�![]() ���ӳɷ�Ӧ��Ӿ۷�Ӧ��������Ӧ��
���ӳɷ�Ӧ��Ӿ۷�Ӧ��������Ӧ��
(4)�����������л���E���״�������Ϊ(F )C6H5-CHO����Ӧ�ߵĻ�ѧ����ʽΪ��![]() ������������������ǣ�
������������������ǣ�![]() ��
��
(5) D��G����ȡ����Ӧ��Ӧ����H�Ļ�ѧ����ʽΪ
 ������������������ǣ�
������������������ǣ�
 ��
��
(6) GΪC6H5-CH=CH2COOH����G������ͬ�����ŵķ����廯���ﻹ�У� (�ڡ��䡢��3��)��
(�ڡ��䡢��3��)�� ��1�֣���4�֣�����������������ǣ�4��
��1�֣���4�֣�����������������ǣ�4��


 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ѿ��г���������������ΪʳƷ���Ӽ���Ϊ���ij���Ѿ���Ʒ���������εĺ�����ͨ���Ծ�����SO2�����ƣ���ij�о�С�����������ʵ�飨��֪��ԭ�ԣ�SO32->I->Cl-��������˵������ȷ����
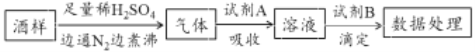
A. ���Ѿ��м��������ε���ҪĿ���Ƿ�ֹ�������������������εĻ�ԭ��
B. ͨ��N2����е�Ŀ����Ϊ�˽��������������Һ��ȫ���ϳ�
C. ���Լ�Aѡ����ˮ�����Լ�B��ѡ��NaOH��Һ
D. ���Լ�Aѡ���Һ���������պ���ҺΪ���ԣ����Լ�B��ѡ��I2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮԼռ����������71%������ʮ�־�Ŀ���DZ������ͼ�ǿ�ˮ��Դ�ۺ����õĹ���ͼ������˵����ȷ( )

A. ���NaCl��Һʱ���������缫,�����ӷ���ʽΪ��2Cl- + 2H2O = 2OH-+ H2��+ Cl2��
B. ���±�м���Cl2���������û����嵥�ʣ��ù��������˻�ԭ�ԣ�Cl- > Br-
C. ʵ����ģ�⺣ˮ��ȡ��ˮ�����г�װ����ֻ�õ���������������ƿ���ƾ��ơ���ƿ
D. Br2 ��SO2��ˮ��Һ������Ӧ�����ӷ���ʽΪ��Br2 + SO2 + 2H2O =4H+ + 2Br- + SO42-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��T��ʱ��A������B���巴Ӧ����C���壬��Ӧ������A��B��CŨ�ȱ仯��ͼ(I)��ʾ�������������������䣬�¶ȷֱ�ΪT1��T2ʱ��B�����������ʱ��Ĺ�ϵ��ͼ(II)��ʾ�����������������ش��������⣺

��1���÷�Ӧ�Ļ�ѧ����ʽΪ________________________
��2���÷�Ӧ��ƽ�ⳣ������ʽΪ__________��T��ʱ��ƽ�ⳣ������ֵΪ(����һλС��)___________��
��3���÷�ӦΪ_________(��������������������)��Ӧ
��4��t1min�ı�����ijһ��������ʹƽ�����淴Ӧ�����ƶ�����_____(����ĸ)
A.���������������䣬����ѹǿ B.����������������䣬ͨ������ϡ������
C.���������������䣬�����¶� D.���������������䣬�����¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ϣʱ�������Ĵ������������Ի�������˼������в��ij�����Ϊ����ѧ��̽��С�齫һ����������·������õ���70%Cu��25%Al��4%Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�����·�ߣ�

�ش��������⣺
(1)�ڢٲ�Cu���ᷴӦ�Ļ�ѧ����ʽΪ_______________________________________��
�õ�����1����Ҫ�ɷ�Ϊ________________��
(2)�ڢڲ�����H2O2�ĵ����ӷ���ʽΪ______________________________________��ʹ��H2O2���ŵ���______________________________________________������pH�������ij����Ļ�ѧʽΪ______��______��
(3)�õڢ۲�����CuSO4��5H2O�Ʊ���ˮCuSO4�ķ�����_______________________��
(4)������2��ȡAl2(SO4)3��18H2O��̽��С����������ַ�����

�������ַ����У����з�����______�����в����з�����ԭ����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������г������ʵ������뻯ѧʽ���Ӧ����
A.�մ���NaHCO3B.������KAlSO4��12H2O
C.̼���(NH4)2CO3D.������CuSO4��5H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ij�¶��£������Ϊ5 L�������У�A��B��C�����������ʵ�������ʱ��仯�Ĺ�ϵ��ͼ��ʾ����÷�Ӧ�Ļ�ѧ����ʽΪ__________��2 s����A��Ũ�ȱ仯����B��Ũ�ȱ仯��ʾ��ƽ����Ӧ���ʷֱ�Ϊ________��________��

��2����0.6 mol X������0.6 mol Y��������2 L�����з�����Ӧ��2X(g)��Y(g)=nZ(g)��2W(g)��2 minĩ������0.2 mol W���������v(Z)Ũ�ȱ仯��ʾ��v(Z)��0.1 mol/(L��min)����
(1)n��_____��
(2)ǰ2 min�ڣ�v(X)��_____��
(3)2 minĩʱY��Ũ��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵����ȷ����
A. ��״���£�22.4 L CCl4�к�CCl4������ΪNA
B. 5.6 g����6.4 gͭ�ֱ���0.1 mol������ȫ��Ӧ��ת�Ƶĵ��������
C. 0.1 mo1��L��1 MgCl2��Һ�к�Cl����Ϊ0.2NA
D. 3.9 g Na2O2 �����к��е���������Ϊ0.2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������·�Ӧ�õ���![]()
![]()
![]()
![]()
![]() �����Ľṹ��ʽ��������
�����Ľṹ��ʽ��������
A. CH3CH(CH2Br)2 B. (CH3)2CBrCH2Br
C. CH3CH2CHBrCH2Br D. CH3CHBrCHBrCH3
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com