
ij���ͭ�������ַ�ˮ��Ҫ������һ�ַ�ˮ�к���CN-���ӣ���һ�ַ�ˮ�к���Cr2O72-���ӣ��ó��ⶨ��ͼ��ʾ�ķ�ˮ�������̡�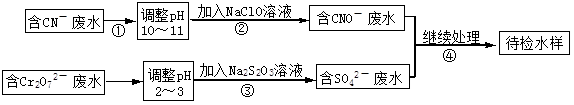 �ش��������⣺
�ش��������⣺
��1������ڷ�����Ӧ�����ӷ���ʽ�ɱ�ʾ���£�aCN��+bClO��+2cOH��=dCNO��+eN2��+fCO32��+bCl��+cH2O���������ӷ���ʽ���ܵ���ƽϵ���ж��飬��ش�
�ٷ���ʽ��e : f��ֵΪ ����ѡ���ţ���
| A��1 | B��1/2 | C��2 | D������ȷ�� |
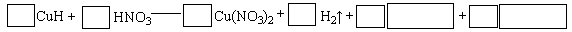
��1����B��6��1.12L
��2��4Cr2O72����3S2O32����26H����6SO42����8Cr3����13H2O
��3����Cu2����2OH����Cu(OH)2��
��Cu(OH)2(s)��S2��(aq)��CuS(s) ��2OH��(aq)
��4����2CuH��2H����Cu��Cu2����2H2��
��6CuH��16HNO3��6Cu(NO3)2��3H2����4NO����8H2O
���������������1�������������غ㣬��֪ѡB�������ݵ���غ��֪Ϊ6
���㣺������ԭ���й�֪ʶ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Т���Ƭ ��NaCl��Һ �۰�ˮ �ܴ��� �ݾƾ� ������ ��H2SO4
��KOH���� ������ ��KAl(SO4)2��12H2O�������ܵ������ �����ڵ���ʵ���____ ___�����ڷǵ���ʵ��� ����������� �����ڼ���� ��������ţ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣��������ʣ� ������ ��Br2 ��Na2O ��Ba(OH)2 ��CO2 ��SO3
��NaCl��Һ ��NaCl ��HCl ��H2SO4
���ڵ���ʵ��� �� ���ڷǵ���ʵ��� ���ܵ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ѧУ�����ĺ�ˮ�и�Ƽ�賤������ˮ�ʶ���ˮˮ���п��ܺ���Fe3����Ba2����K����H+��NO3����Cl-��CO32-��SO42-���ӡ�Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺
��ȡˮ����ϸ�۲죬��������һ״̬��
����pH��ֽ�ⶨ��ˮ��pH����ֽ�Ժ�ɫ��
����ˮ���е���KSCN��Һ���ʺ�ɫ��
����ˮ���е���ϡ���ᣬ�д�����ɫ�����������ټ�ϡ���ᣬ��ɫ��������ʧ��
��1���ɴ˿�֪������ˮ�п϶����е�������_________���϶�û�е�������_________��
��2�� ��Ƽ�賤�Ŀ���ԭ����ˮ�к��н϶��_____________���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ֱ���һ���Լ������������л�����������ʳ�ȥ��������Ϊ��������ʣ�
| ���� | �������Լ� | �й����ӷ���ʽ |
| ��1��HNO3(H2SO4) | | |
| ��2��Cu(Fe) | | |
| ��3��NaCl(Na2CO3) | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ɫ��ҺX����Na+��Ag+��Ba2+��Al3+��AlO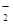 ��MnO
��MnO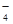 ��CO
��CO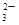 ��SO
��SO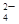 �е�������������ɣ�ȡ��Һ������ͼʵ�飺
�е�������������ɣ�ȡ��Һ������ͼʵ�飺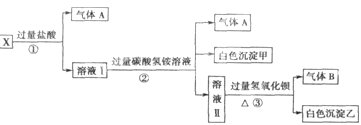
��1����ɫ�������� ��
��2��X��Һ��һ�����ڵ������� ��
��3����ɫ��������һ���У� �������� ��
��4����������������A������������Bͨ��ˮ�У�д����Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ����K+��Cu2+��Fe3+��Al3+��Fe2+��Cl����CO2��3��OH����NO��3��SO2��4�еļ��֣���֪����Һ�и��������ʵ���Ũ�Ⱦ�Ϊ0��2mol/L��������ˮ�ĵ��뼰���ӵ�ˮ�⣩��Ϊȷ������Һ�к��е����ӣ��ֽ��������µIJ�����
I��ȡ������Һ������KSCN��Һʱ�����Ա仯��
��ȡԭ��Һ����BaCl2��Һ���а�ɫ�������ɣ�
����ȡ��Һ�������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������û�����ӡ�
���ƶϣ�
��l�����ɲ���I������ɵó��Ľ�����____��
��2�����ɲ���������ɵó��Ľ�����____��
��3���ɲ���������ɵó���Һ��һ�����е���������____��д���ӷ��ţ����������з�����Ӧ�Ļ�ѧ����ʽΪ____��
��4��������������������֪������Ϊԭ��Һ���������������____�֡�
��5����ȡl00mLԭ��Һ������������NaOH��Һ����ַ�Ӧ����ˣ�ϴ�ӣ����������أ��õ��Ĺ�������Ϊ g��д���˹������漰������ԭ��Ӧ�Ļ�ѧ����ʽ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij������ˮ��Һ�����ܺ������������е������֣�K+��Al3+��Fe3+��Mg2+��Ba2+��NH4+��Cl-��CO32-��SO42-���ֱַ�ȡ100mL�����ȷ���Һ��������ʵ�飺
�ٵ�һ�ݼӹ���NaOH��Һ����ȣ��ռ���0.02mol���壬�������ɣ�ͬʱ�õ���Һ�ס�
�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02g���塣
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65g���塣
����ʵ��ش��������⣺
��1���ɢٿ�֪���ڵ�����Ϊ ��Ũ���� mol��L-1��
�ɢڿ�֪���ڵ�����Ϊ ��Ũ���� mol��L-1��
�ɢۿ�֪���ڵ�����Ϊ ��Ũ���� mol��L-1��
��2������Һ��һ�������ڵ������� �������ӷ��ţ���
��3��ijͬѧͨ��������Ϊ����Һ��һ������K+������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���������������ļӿ죬�����С��װʳƷ�ѱ��㷺���ܡ�Ϊ���ӳ�ʳƷ�ı����ڣ���ֹʳƷ�ܳ�����֬ʳƷ�������ʣ��ڰ�װ����Ӧ����Ļ�ѧ������
| A����ˮ����ͭ������ | B���轺���������� |
| C��ʳ�Ρ��������� | D����ʯ�ҡ�ʳ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com