
ȡ��ѧʽΪMZ�Ļ�ɫ��ĩ״�������������ʵ�顣��MZ������̼�۳�ֻ�ϣ�ƽ���ڷ�Ӧ��a�У���bƿ��ʢ��������ʯ��ˮ����ͼ����������
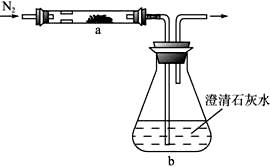
ʵ�鿪ʼʱ����ͨ�뵪������һ��ʱ����ȷ�Ӧ��a���۲쵽���ڷ������ҷ�Ӧ���������������ɡ�ͬʱ��bƿ����Һ�г��ְ�ɫ���ǡ�����Ӧ��ȫ��ֹͣ���ȣ��Լ���ͨ������ֱ����Ӧ����ȴ����ʱ�����е����������̳�����ɫ�������������������ش����⣺
��1��Ԫ��Z����������������
��2��ֹͣ����ǰ�Ƿ���Ҫ�ȶϿ�a��b�����Ӵ���Ϊʲô��
��������������������������������������������������������������������
��3����Ӧ��a�з��������з�Ӧ�Ļ�ѧ����ʽ��
��������������������������������������������������������������������
��4����ʵ���β���Ƿ��账�������账������ش���δ������粻�账������˵�����ɡ�
��������������������������������������������������������������������������
���𰸡�
��1����
��2������Ҫ����ΪN2����ͨ�룬bƿ��Һ���ᵹ����a��
��3��MO+C M+CO�� MO+CO
M+CO�� MO+CO M+CO2
M+CO2
CO2+C 2CO 2MO+C
2CO 2MO+C 2M+C
2M+C O2��
O2��
��4���账��������CO��������һ�����ȵ�װ��CuO��ĩ�ķ�Ӧ��
����������1����ѧʽΪMZ�Ļ�����������̼�۳�ֻ�ϼ��Ⱥ�Ӧ����������������ʯ��ˮ���յð�ɫ��������֪��������CO2������֪MZ������Ԫ�أ���ZΪ��Ԫ�ء�
��2����ֹͣ����ǰ����Ҫ�ȶϿ�a��b�����Ӵ�����Ϊ�ڲ���ͨ��N2������£�������������
��3���ɷ����ķ�Ӧ��MO+C M+CO�� CO+MO
M+CO�� CO+MO M+CO2 CO2+C
M+CO2 CO2+C 2CO 2MO+C
2CO 2MO+C 2M+CO2��
2M+CO2��
��4����ʵ��β���к���CO����Ҫβ��������������һ�����ȵ�ʢ��CuO��ĩ�ķ�Ӧ�ܣ����Ҳർ�ܿڼ�һ��ȼ�ľƾ��ƣ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�������Ԥ��ʵ��Ŀ�Ļ�����ʵ�����һ�µ���
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ij�����������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ��ζ���� | ˵���ü�����K2CO3 |
| B | ��������FeCl3��MgCl2��Һ�м�������Mg(OH)2��ĩ������һ��ʱ������ | ��ȥMgCl2��Һ������FeCl3 |
| C | �����£���Na2CO3��Һ�м�����BaSO4��ĩ�����ˣ���ϴ���ij����м�ϡ���ᣬ�����ݲ��� | ˵��������K sp(BaCO3)��K sp(BaSO3) |
| D | C2H5OH��Ũ����170�湲�ȣ��Ƶõ�����ͨ������KMnO4��Һ | �����Ƶõ������Ƿ�Ϊ��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʵ��С����������װ�ã����̶ֹ�װ���ԣ��Ʊ������ƣ�Ca3N2������̽����ʵ��ʽ��

��1����ͼ���Ӻ�ʵ��װ�á����װ�õ������ԣ�������_____________________
______________________________________________________________________��
��2����Ӧ������ĩ�˵��ܱ���ʼ�ղ����Թ�A��ˮ�У�Ŀ����_______________________
_______________________________________��
��3���Ʊ������ƵIJ��������ǣ��ٴ���K��ͨ��N2���ڵ�ȼ�ƾ��ƣ����з�Ӧ���۷�Ӧ������__________________���ܲ��װ�ã�ȡ�����
��4�����ݼ�¼���£�
| �մ�������m0/g | ������Ƶ�����m1/g | ��������������m2/g |
| 14.80 | 15.08 | 15.15 |
�ټ���õ�ʵ��ʽCaxN2������x��_______________________��
����ͨ���N2�л�������O2����Ƚ�x��3�Ĵ�С���������ж����ݣ�_________________
___________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ע�⣺����Ϊ�ֲ��⣬��A��B���⣬��������ѡһ�⡣�����������һ�ɰ�A��Ʒ֡�A���ʺ�ʹ�ö��ڿθ��½̲ĵĿ������B���ʺ�ʹ��һ�ڿθĽ̲ĵĿ������
��A������ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ����

| ʵ����� | ����Ͳ������ | ����Ͳ������ | ����Ͳ������ |
| 1 | 10 mL FeSO4��Һ | 10 mL NH3 | ���ɰ�ɫ���������ɫ |
| 2 | 20 mL H2S | 10 mL SO2 | |
| 3 | 30 mL NO2����Ҫ�� | 10 mL H2O(l) | ʣ����ɫ���壬�����Զ�����ѹ�� |
| 4 | 15 mL Cl2 | 40 mL NH3 |
�Իش��������⣺
��1��ʵ��1�У��������ձ�Ϊ___________ɫ��д��������ɫ�Ļ�ѧ����ʽ_______________��
��2��ʵ��2����Ͳ�ڵ������ǣ���________���ɣ�����___________�ƶ�������⡱�����ڡ�����������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��__________��Һ�С�
��3��ʵ��3�У����е�30 mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������__________��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽ_______________________________��
��4��ʵ��4�У���֪��3Cl2+2NH3 N2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ___________�������Ͳ��ʣ����������ԼΪ______________mL��
N2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ___________�������Ͳ��ʣ����������ԼΪ______________mL��
��B��ijʵ��С��������װ�ý����Ҵ���������ʵ�顣

��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ
_____________________________________________________________________
_____________________________________________________________________��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��________��Ӧ��
��2����������ˮԡ���ò���ͬ��
��������____________________���ҵ�������_____________________��
��3����Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�������____________________________������ƿ���ռ������������Ҫ�ɷ���______________��
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����__________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���______________����д��ĸ����
a.�Ȼ�����Һ  b.��
b.��
c.̼��������Һ d.���Ȼ�̼
Ȼ����ͨ��_____________����ʵ��������ƣ����ɳ�ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ����һ��Ƭ����Ԫ�صĺ�����ij����С������������ַ�����������ʵ�飨��������Ϊ���ƽ��ʵ��ⶨ�����ƽ��ֵ����
����һ����a g��Ƭ��ȫ�ܽ��ڹ���ϡ�����У�����������������Ϊ580mL����״������
���������� g��Ƭ��ȫ�ܽ��ڹ���ϡ�����У�����Ӧ��õ�����Һ��0.02000mol��L-1
g��Ƭ��ȫ�ܽ��ڹ���ϡ�����У�����Ӧ��õ�����Һ��0.02000mol��L-1
��KMnO4��Һ�ζ����ﵽ�յ�ʱ������25.00mL KMnO4��Һ��
��ش��������⣺
��1����ƽ����Ļ�ѧ����ʽ��
��KMnO4+��FeSO4+��H2SO4=��Fe2(SO4)3+��MnSO4+��K2SO4+��H2O
��2���ڵζ�ʵ���в���ѡ����������������ʽ�ζ��ܣ���������������������������
��3�����ݷ���һ�ͷ������ⶨ�Ľ�����㣬��Ƭ������������������Ϊ��������������
�����������������������ԭ��������55.9�ƣ�
��4�����ų�ʵ�������Ͳ�����Ӱ�����أ��Զ��������ַ����ⶨ�����ȷ�������жϺͷ�����
�ٷ���һ�������������ȷ������ȷ������һ��ȷ����������������������������
�ڷ������������������ȷ������ȷ������һ��ȷ����������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���ܴﵽԤ��Ŀ�ĵ���
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��SO2ͨ������KMnO4��Һ�� | ֤��SO2���������� |
| B | ��Cl2ͨ��NaBr��Һ�� | �Ƚ��������������ǿ�� |
| C | ��ͭ��Ũ���ᷴӦ���ɵ������ռ����ñ�ˮ�������ȴ���� | �о��¶ȶԻ�ѧƽ���Ӱ�� |
| D | �ֱ���2֧�Թ��м�����ͬ�����ͬŨ�ȵ�H2O2��Һ����������1֧��������MnO2 | �о�������H2O2�ֽ����ʵ�Ӱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ӽ���ij��÷��������֣�
| ���鷽�� | ������ | ��ɫ�� | ���巨 |
| ���� | ��Ӧ���г����������ܽ� | ��Ӧ������ɫ�仯 | ��Ӧ����������� |
�������Ӽ���ķ�������������
A NH4+�����巨 B I����������
C Fe3+����ɫ�� D Ca2+�����巨
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ�ж�����;��������������ɫ��������������ȡ�Ϊ��ô���������ͭ��̽�������ʣ�ijͬѧ�ù�ҵ����ͭ(����������������)��������ʵ�飺
���Ʊ�����ͭ
��ҵCuSO4
 CuSO4��Һ
CuSO4��Һ CuSO4��5H2O��������CuO
CuSO4��5H2O��������CuO
�ٲ���I��Ŀ���dz����������ʡ������� ��
�ڲ�����Ŀ���dz����������ǣ��μ�H2O2��Һ���Լ��ȣ���Fe2+ת����ȫ����������Cu2(OH)2CO3��ĩ�����裬�Կ�����ҺpH=3.5���������һ��ʱ�䣬���ˣ���ϡ�����ữ��Һ��pH=1��������ҺpH=3.5��ԭ���� ��
�۲�����Ŀ���ǵõ�CuSO4��5H2O���塣������ �����ˣ�ˮԡ���Ⱥ�ɡ�ˮԡ���ȵ��ص��� ��
��̽������ͭ������
��ȡA��B��֧�Թܣ���A���ȼ�������CuO��ĩ���ٷֱ���A��B�м���������3% H2O2��Һ��ֻ�۲쵽A���д������ݡ������� ��
��Ϊ̽���Թ�A�з�Ӧ�����ʣ��ռ����岢�ⶨ����������ʵ�������У�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����豸����ʱ,����ѧ��ת��Ϊ���ܵ���(����)
| A | B | C | D |
|
|
|
|
|
| ��̫���ܵ�� | ����ӵ�� | ̫���ܼ����� | ȼ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com