
ЁОЬтФПЁПЯжгаЪвЮТЯТШмжЪХЈЖШОљЮЊ![]() ЕФМИжжШмвКЃКЂйбЮЫсЁЂЂкСђЫсЁЂЂлДзЫсЁЂЂмСђЫсяЇЁЂЂнАБЫЎЁЂЂоЧтбѕЛЏФЦШмвКЃЌЛиД№ЯТСаЮЪЬтЃК
ЕФМИжжШмвКЃКЂйбЮЫсЁЂЂкСђЫсЁЂЂлДзЫсЁЂЂмСђЫсяЇЁЂЂнАБЫЎЁЂЂоЧтбѕЛЏФЦШмвКЃЌЛиД№ЯТСаЮЪЬтЃК
(1)дкЂмШмвКжаЃЌИїРызгХЈЖШДѓаЁЫГађЮЊЃК____________
(2)НЋЂлЁЂЂоЛьКЯКѓЃЌШєШмвКГЪЯжжаадЃЌдђЯћКФСПШмвКЕФЬхЛ§ЮЊЂл____________Ђо(ЬюЁА>ЁБЁЂЁА=ЁБЛђЁА<ЁБ)ЃЌШмвКжаЕФРызгХЈЖШгЩДѓЕНаЁЕФЫГађЮЊ____________
(3)дкГЃЮТЯТЃЌНЋ100mLЕФЂкгы100mLЕФЂоШмвКЛьКЯКѓ(МйЩшЛьКЯКѓШмвКЕФЬхЛ§ЮЊЛьКЯЧАШмвКЕФЬхЛ§жЎКЭ)ЃЌШмвКЕФpH=____________(вбжЊ![]() )
)
(4)дкГЃЮТЯТЃЌСљжжвКЬхЕФpHгЩДѓЕНаЁЕФЫГађЪЧ____________
(5)ШєНЋЂлШмвККЭЂоШмвКАДЬхЛ§БШ2:1ЛьКЯКѓШмвКГЪЫсадЃЌдђЛьКЯКѓШмвКжа![]() __________
__________![]() (ЬюЁА>ЁБЁЂЁА=ЁБЛђЁА<ЁБ)
(ЬюЁА>ЁБЁЂЁА=ЁБЛђЁА<ЁБ)
(6)ГЃЮТЯТНЋЂлШмвКМгЫЎЯЁЪЭЙ§ГЬжаЃЌЯТСаБэДяЪНЕФЪ§ОнвЛЖЈБфДѓЕФЪЧ______
AЁЂ![]() BЁЂ
BЁЂ![]() CЁЂ
CЁЂ![]() DЁЂ
DЁЂ![]()
ЁОД№АИЁПc(NH4ЃЋ)>c(SO42Ѓ)>c(HЃЋ)>c(OHЃ) > c(NaЃЋ)=c(CH3COOЃ)>c(HЃЋ)=c(OHЃ) 3.3 Ђо>Ђн>Ђм>Ђл>Ђй>Ђк > B D
ЁОНтЮіЁП
(1)ЂмЮЊ(NH4)2SO4ЃЌ![]() ЫЎНтШмвКГЪЫсадЃЌЕЋЫЎНтЪЧЮЂШѕЕФЃЌВЛЛсДяЕН50%ЃЌШмвКжаc(
ЫЎНтШмвКГЪЫсадЃЌЕЋЫЎНтЪЧЮЂШѕЕФЃЌВЛЛсДяЕН50%ЃЌШмвКжаc(![]() )ЃОc(
)ЃОc(![]() )ЃЌc(HЃЋ)ЃОc(OH)ЃЌЫљвдРызгХЈЖШДѓаЁЮЊЃКc(
)ЃЌc(HЃЋ)ЃОc(OH)ЃЌЫљвдРызгХЈЖШДѓаЁЮЊЃКc(![]() )ЃОc(
)ЃОc(![]() )ЃОc(HЃЋ)ЃОc(OH)ЃЌ
)ЃОc(HЃЋ)ЃОc(OH)ЃЌ
ЙЪД№АИЮЊЃКc(![]() )ЃОc(
)ЃОc(![]() )ЃОc(HЃЋ)ЃОc(OH)ЃЛ
)ЃОc(HЃЋ)ЃОc(OH)ЃЛ
(2)ЂлЮЊCH3COOHЃЌЂоЮЊNaOHЃЌШєЧЁКУЗДгІЩњГЩCH3COONaЃЌCH3COONaЫЎНтШмвКЮЊМюадЃЌЮЊЪЙШмвКГЪзнжаадашвЊЩдЙ§СПЕФCH3COOHЃЌЫљвдЯћКФСПШмвКЕФЬхЛ§ЮЊЂлЃОЂоЃЌШмвКЮЊжаадЃЌc(HЃЋ)ЃНc(OH)ЃЌИљОнЕчКЩЪиКуЃКc(NaЃЋ)ЃЋc(HЃЋ)ЃНc(OH)ЃЋc(CH3COO)ЃЌЫљвдc(NaЃЋ)ЃНc(CH3COO)ЃЌдђШмвКжаРызгХЈЖШДѓаЁЙиЯЕЮЊЃКc(NaЃЋ)ЃНc(CH3COO)ЃОc(HЃЋ)ЃНc(OH)ЃЌ
ЙЪД№АИЮЊЃКЃОЃЛc(NaЃЋ)ЃНc(CH3COO)ЃОc(HЃЋ)ЃНc(OH)ЃЛ
(3)ГЃЮТЯТЃЌНЋ100mLЕФЂкгы100mLЕФЂоШмвКЛьКЯЃЌЗЂЩњЗДгІЃКH2SO4ЃЋ2NaOHЈTNa2SO4ЃЋ2H2OЃЌЕШЬхЛ§ЕШХЈЖШЗДгІЃЌЫсЙ§СПЃЌдђЗДгІКѓШмвКжаc(HЃЋ)ЃН![]()
mol/LЃН5ЁС104mol/LЃЌдђШмвКpHЃНlgc(HЃЋ)ЃН3.3ЃЌ
ЙЪД№АИЮЊЃК3.3ЃЛ
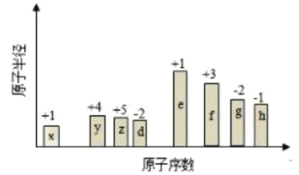
(4)СђЫсЮЊЖўдЊЫсЃЌЭЌХЈЖШЕФЬѕМўЯТЃЌСђЫсЫсадЧПгкбЮЫсЃЌДзЫсЮЊШѕЕчНтжЪЃЌЫсадШѕгкбЮЫсЃЌСђЫсяЇЫЎНтЮЊЫсадЃЌЫсадИќШѕЃЌАБЫЎЮЊШѕМюЃЌЧтбѕЛЏФЦЮЊЧПМюЃЌЫсаддНШѕЃЌpHжЕдНДѓЃЌЫљвдЮхжжвКЬхЕФpHгЩДѓЕНаЁЕФЫГађЪЧЃКЂо>Ђн>Ђм>Ђл>Ђй>ЂкЃЌ
ЙЪД№АИЮЊЃКЂо>Ђн>Ђм>Ђл>Ђй>ЂкЃЛ
(5)НЋЂлШмвККЭЂоШмвКАДЬхЛ§БШ2ЃК1ЛьКЯКѓШмвКГЪЫсадЃЌЯрЕБгкЕШСПЕФCH3COOHКЭCH3COONaЕФЬѕМўЯТШмвКЮЊЫсадЃЌдђДзЫсЕФЕчРыГЬЖШДѓгкЫЎНтГЬЖШЃЌдђЛьКЯКѓШмвКжаc(CH3COO)ЃОc(CH3COOH)ЃЌ
ЙЪД№АИЮЊЃКЃОЃЛ
(6)AЃЎЯЁЪЭЙ§ГЬжаЃЌn(HЃЋ)діДѓЃЌЕЋШмвКЬхЛ§діМгИќДѓЃЌећЬхРДЫЕc(HЃЋ)МѕаЁЃЌЙЪAВЛбЁЃЛ
BЃЎЯЁЪЭЙ§ГЬжаc(HЃЋ)МѕаЁЃЌдђc(OH)ЃН![]() діДѓЃЌЙЪBбЁЃЛ
діДѓЃЌЙЪBбЁЃЛ
CЃЎKwЃН![]() жЛЫцЮТЖШИФБфЖјИФБфЃЌЙЪCВЛбЁЃЛ
жЛЫцЮТЖШИФБфЖјИФБфЃЌЙЪCВЛбЁЃЛ
D.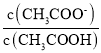 ЃЌЯЁЪЭЙ§ГЬжаc(HЃЋ)МѕаЁЃЌдђ
ЃЌЯЁЪЭЙ§ГЬжаc(HЃЋ)МѕаЁЃЌдђ діДѓЃЌЙЪDбЁЃЌ
діДѓЃЌЙЪDбЁЃЌ
ЙЪД№АИЮЊЃКBDЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЪЕбщЪЧЛЏбЇбаОПЕФЛљДЁЃЌЯТЭМЙигкИїЪЕбщзАжУ(МаГжзАжУвбТдШЅ)ЕФа№ЪіЃЌе§ШЗЕФЪЧ( )
A.  ЮќЪеHClЦјЬхЃЌВЂЗРжЙЕЙЮќ
ЮќЪеHClЦјЬхЃЌВЂЗРжЙЕЙЮќ
B. ![]() зМШЗСПШЁвЛЖЈЬхЛ§K2Cr2O7БъзМШмвК
зМШЗСПШЁвЛЖЈЬхЛ§K2Cr2O7БъзМШмвК
C.  жЦБИЬМЫсЧтФЦ
жЦБИЬМЫсЧтФЦ
D.  еєИЩFeCl3ШмвКжЦБИЮоЫЎFeC13
еєИЩFeCl3ШмвКжЦБИЮоЫЎFeC13
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФмдДЪЧЙњУёОМУЗЂеЙЕФживЊЛљДЁЃЌЮвЙњФПЧАЪЙгУЕФФмдДжївЊЪЧЛЏЪЏШМСЯЁЃ
(1)вбжЊЃК ![]()
![]()
![]()
дђУКЦјЛЏжївЊЗДгІ![]() ________________
________________
(2)вбжЊ![]() ЕФе§ЗДгІЫйТЪЮЊ
ЕФе§ЗДгІЫйТЪЮЊ![]() ЃЌФцЗДгІЫйТЪЮЊ
ЃЌФцЗДгІЫйТЪЮЊ![]() ЃЌkЮЊЫйТЪГЃЪ§ЁЃ2500KЪБЃЌ
ЃЌkЮЊЫйТЪГЃЪ§ЁЃ2500KЪБЃЌ ![]() ЃЌдђИУЮТЖШЯТЕФЗДгІЦНКтГЃЪ§K=_________________ ЁЃ
ЃЌдђИУЮТЖШЯТЕФЗДгІЦНКтГЃЪ§K=_________________ ЁЃ
(3)МзДМжЦМзУбЕФгаЙиЗДгІЮЊЃК ![]() вЛЖЈЮТЖШЯТЃЌдкШ§ИіШнЛ§ОљЮЊ1.0 LЕФКуШнУмБеШнЦїжаЗЂЩњИУЗДгІЁЃ
вЛЖЈЮТЖШЯТЃЌдкШ§ИіШнЛ§ОљЮЊ1.0 LЕФКуШнУмБеШнЦїжаЗЂЩњИУЗДгІЁЃ
ШнЦїБрКХ | ЮТЖШЃЏЁц | Ц№ЪМЮяжЪЕФСПЃЏmol | ЦНКтЮяжЪЕФСП/mol | |
CH3OH | CH3OCH3 | H2O | ||
I | 387 | 0. 20 | x | |
II | 387 | 0. 40 | y | |
Ђѓ | 207 | 0. 20 | 0. 090 | 0. 090 |
![]() ________________.
________________.
ЂквбжЊ![]() ЪБИУЗДгІЕФЛЏбЇЦНКтГЃЪ§K=4ЁЃИУЮТЖШЯТЃЌШєЦ№ЪМЪБЯђШнЦїIжаГфШы0.10mol
ЪБИУЗДгІЕФЛЏбЇЦНКтГЃЪ§K=4ЁЃИУЮТЖШЯТЃЌШєЦ№ЪМЪБЯђШнЦїIжаГфШы0.10mol ![]() ЃЌдђЗДгІНЋЯђ_________ЃЈЬюЁАе§ЁБЛђЁАФцЁБЃЉЗДгІЗНЯђНјааЁЃ
ЃЌдђЗДгІНЋЯђ_________ЃЈЬюЁАе§ЁБЛђЁАФцЁБЃЉЗДгІЗНЯђНјааЁЃ
ЂлШнЦїЂђжаЗДгІДяЕНЦНКтКѓЃЌШєвЊНјвЛВНЬсИпМзУбЕФВњТЪЃЌПЩвдВЩШЁЕФДыЪЉЮЊ______________ЃЈЬюађКХЃЉ
A.Щ§ИпЮТЖШ B.ЦфЫћЬѕМўВЛБфЃЌдіМг![]() ЕФЮяжЪЕФСП
ЕФЮяжЪЕФСП
C.НЕЕЭЮТЖШ D.БЃГжЦфЫћЬѕМўВЛБфЃЌЭЈШыФЪЦј
(4)ЮЊМѕЩйЮэіВЁЂНЕЕЭДѓЦјжагаКІЦјЬхКЌСПЃЌбаОПЛњЖЏГЕЮВЦјжа![]() МА
МА![]() ЕФХХЗХСПвтвхжиДѓЁЃЛњЖЏГЕЮВЦјЮлШОЮяЕФКЌСПгыПеЃЏШМБШЃЈПеЦјгыШМгЭЦјЕФЬхЛ§БШЃЉЕФБфЛЏЙиЯЕЪОвтЭМШчЭМЫљЪОЃК
ЕФХХЗХСПвтвхжиДѓЁЃЛњЖЏГЕЮВЦјЮлШОЮяЕФКЌСПгыПеЃЏШМБШЃЈПеЦјгыШМгЭЦјЕФЬхЛ§БШЃЉЕФБфЛЏЙиЯЕЪОвтЭМШчЭМЫљЪОЃК

ЂйЕБПеЃЏШМБШДяЕН15Кѓ![]() МѕЩйЕФдвђПЩФмЪЧ__________ЃЈЬюзжФИЃЉЁЃ
МѕЩйЕФдвђПЩФмЪЧ__________ЃЈЬюзжФИЃЉЁЃ
a.ЗДгІ![]() ЪЧЮќШШЗДгІ
ЪЧЮќШШЗДгІ
b.ЕБПеЃЏШМБШДѓИЩ15КѓЃЌШМгЭЦјШМЩеЗХГіЕФШШСПЯргІМѕЩй
ЂкЫцПеЃЏШМБШдіДѓЃЌCOКЭ![]() ЕФКЌСПМѕЩйЕФдвђЪЧ______ЁЃ
ЕФКЌСПМѕЩйЕФдвђЪЧ______ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯђ1LFeBr2ШмвКжаЃЌЭЈШы1ЃЎ12LЃЈБъЬЌЯТЃЉЕФCl2ЃЌВтЕУШмвКжаcЃЈBr-ЃЉЃН3cЃЈCl-ЃЉЃЌЗДгІЙ§ГЬжаШмвКЕФЬхЛ§БфЛЏВЛМЦЃЌдђЯТСаЫЕЗЈжае§ШЗЕФЪЧ
A. дШмвКЕФХЈЖШЮЊ0ЃЎ1mol/L B. ЗДгІКѓШмвКжаcЃЈFe3+ЃЉЃН0ЃЎ1mol/L
C. ЗДгІКѓШмвКжаcЃЈFe3+ЃЉЃНcЃЈFe2+ЃЉ D. дШмвКжаcЃЈBr-ЃЉЃН0ЃЎ4mol/L
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЃЈ10ЗжЃЉЧІаюЕчГиЪЧГЃгУЕФЛЏбЇЕчдДЃЌЦфЕчМЋВФСЯЗжБ№ЪЧPbКЭPbO2ЃЌЕчНтвКЮЊЯЁСђЫсЁЃЗХЕчЪБЃЌИУЕчГизмЗДгІЪНЮЊЃКPbЃЋPbO2ЃЋ2H2SO4 ![]() 2PbSO4ЃЋ2H2OЁЃЧыИљОнЩЯЪіЧщПіХаЖЯЃК
2PbSO4ЃЋ2H2OЁЃЧыИљОнЩЯЪіЧщПіХаЖЯЃК
ЃЈ1ЃЉИУаюЕчГиЕФИКМЋВФСЯЪЧ_________ЃЌЗХЕчЪБЗЂЩњ_________ЃЈЬюЁАбѕЛЏЁБЛђЁАЛЙдЁБЃЉЗДгІЁЃ
ЃЈ2ЃЉИУаюЕчГиЗХЕчЪБЃЌЕчНтжЪШмвКЕФЫсад_________ЃЈЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБЃЉЃЌЕчНтжЪШмвКжавѕРызгвЦЯђ_________ЃЈЬюЁАе§ЁБЛђЁАИКЁБЃЉМЋЁЃ
ЃЈ3ЃЉвбжЊСђЫсЧІЮЊВЛШмгкЫЎЕФАзЩЋЙЬЬхЃЌЩњГЩЪБИНзХдкЕчМЋЩЯЁЃЪдаДГіИУЕчГиЗХЕчЪБЃЌе§МЋЕФЕчМЋЗДгІ_______________________________________ЃЈгУРызгЗНГЬЪНБэЪОЃЉЁЃ
ЃЈ4ЃЉЧтбѕШМСЯЕчГиОпгаЦєЖЏПьЁЂаЇТЪИпЕШгХЕуЃЌЦфФмСПУмЖШИпгкЧІаюЕчГиЁЃШєЕчНтжЪЮЊKOHШмвКЃЌдђЧтбѕШМСЯЕчГиЕФИКМЋЗДгІЪНЮЊ____________________ЁЃИУЕчГиЙЄзїЪБЃЌЭтЕчТЗУПСїЙ§1ЁС103 mol eЃЃЌЯћКФБъПіЯТбѕЦј_________m3ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЧыНтД№ЯТСагыЕААзжЪгаЙиЕФЬтФПЃК
ЃЈ1ЃЉМІЕАИЏРУЪБЃЌГЃЮХЕНгаГєМІЕАЦјЮЖЕФЦјЬхЃЌИУЦјЬхжажївЊКЌга___________________ЃЈЬюЛЏбЇЪНЃЉЃЌЫЕУїЕААзжЪжаКЌга___________________ЃЈЬюдЊЫиУћГЦЃЉдЊЫиЁЃ
ЃЈ2ЃЉЮѓЪГжиН№ЪєбЮЛсжаЖОЃЌетЪЧвђЮЊ___________________ЁЃ
ЃЈ3ЃЉХЈЯѕЫсНІдкЦЄЗєЩЯЃЌЪЙЦЄЗєГЪЯж___________________ЩЋЃЌетЪЧгЩгкХЈЯѕЫсКЭЕААзжЪЗЂЩњСЫ___________________ЗДгІЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЬМШШЛЙдЗЈЙуЗКгУгкКЯН№МАВФСЯЕФжЦБИЁЃЛиД№ЯТСаЮЪЬтЃК
(1)вЛжжжЦБИЕЊбѕЛЏТСЕФЗДгІдРэЮЊ23Al2O3ЃЋ15CЃЋ5N2=2Al23O27N5ЃЋ15CO ЃЌВњЮяAl23O27N5жаЕЊЕФЛЏКЯМлЮЊ______ЃЌИУЗДгІжаУПЩњГЩ1 mol Al23O27N5ЃЌзЊвЦЕФЕчзгЪ§ЮЊ________NAЁЃ
(2)ецПеЬМШШвБТСЗЈАќКЌКмЖрЗДгІЃЌЦфжаЕФШ§ИіЗДгІШчЯТЃК
Ђё.Al2O3(s)ЃЋ3C(s)=Al2OC(s)ЃЋ2CO(g)ЁЁІЄH1
Ђђ.2Al2OC(s)ЃЋ3C(s)=Al4C3(s)ЃЋ2CO(g)ЁЁІЄH2
Ђѓ.2Al2O3(s)ЃЋ9C(s)=Al4C3(s)ЃЋ6CO(g)ЁЁІЄH3
ЂйІЄH3ЃН_________(гУІЄH1ЁЂІЄH2БэЪО)ЁЃ
ЂкAl4C3ПЩгызуСПбЮЫсЗДгІжЦБИвЛжжЬўЁЃИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ________________ЁЃ
(3)ЯТСаЪЧЬМШШЛЙдЗЈжЦУЬКЯН№ЕФШ§ИіЗДгІЃЌCOгыCO2ЦНКтЗжбЙБШЕФздШЛЖдЪ§жЕгыЮТЖШЕФЙиЯЕШчЭМЫљЪО(вбжЊKpЪЧгУЦНКтЗжбЙДњЬцХЈЖШМЦЫуЫљЕУЕФЦНКтГЃЪ§)ЁЃ
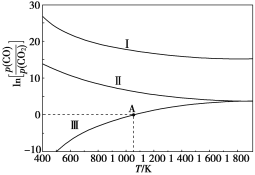
Ђё.Mn3C(s)ЃЋ4CO2(g)![]() 3MnO(s)ЃЋ5CO(g)ЁЁЁЁЁЁЁЁKp(Ђё)
3MnO(s)ЃЋ5CO(g)ЁЁЁЁЁЁЁЁKp(Ђё)
Ђђ.Mn(s)ЃЋCO2(g)![]() MnO(s)ЃЋCO(g)ЁЁЁЁЁЁЁЁЁЁЁЁKp(Ђђ)
MnO(s)ЃЋCO(g)ЁЁЁЁЁЁЁЁЁЁЁЁKp(Ђђ)
Ђѓ.Mn3C(s)ЃЋCO2(g)![]() 3Mn(s)ЃЋ2CO(g)ЁЁЁЁЁЁЁЁKp(Ђѓ)
3Mn(s)ЃЋ2CO(g)ЁЁЁЁЁЁЁЁKp(Ђѓ)
ЂйІЄH>0ЕФЗДгІЪЧ____(ЬюЁАЂёЁБЁАЂђЁБЛђЁАЂѓЁБ)ЁЃ
Ђк1 200 KЪБЃЌдквЛЬхЛ§ЮЊ2 LЕФКуШнУмБеШнЦїжага17.7 g Mn3C(s)КЭ0.4 mol CO2ЃЌжЛЗЂЩњЗДгІЂёЃЌ5 min КѓДяЕНЦНКтЃЌДЫЪБCOЕФХЈЖШЮЊ0.125 mol/LЃЌдђ0ЁЋ5 minФкv(CO2)ЃН_________ЁЃ
ЂлдквЛЬхЛ§ПЩБфЕФУмБеШнЦїжаМгШывЛЖЈСПЕФMn(s)ВЂГфШывЛЖЈСПЕФCO2(g)ЃЌжЛЗЂЩњЗДгІЂђЃЌЯТСаФмЫЕУїЗДгІЂђДяЕНЦНКтЕФЪЧ____(ЬюзжФИ)ЁЃ
A.ШнЦїЕФЬхЛ§ВЛдйИФБфЁЁЁЁB.ЙЬЬхЕФжЪСПВЛдйИФБфЁЁЁЁC.ЦјЬхЕФзмжЪСПВЛдйИФБф
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЫцдзгађЪ§ЕФЕндіЃЌАЫжжЖЬжмЦкдЊЫиЕФдзгАыОЖЕФЯрЖдДѓаЁЁЂзюИпе§МлЛђзюЕЭИКМлЕФБфЛЏШчЯТЭМЫљЪОЃЌЯТСаЗжЮіе§ШЗЕФЪЧЃЈ ЃЉ
A.![]() ЁЂ
ЁЂ![]() ЕФМђЕЅРызгАыОЖДѓаЁЃК
ЕФМђЕЅРызгАыОЖДѓаЁЃК![]()
B.дЊЫиЕФН№ЪєадЃК![]()
C.дЊЫиЕФЗЧН№ЪєадЃК![]()
D.![]() ЁЂ
ЁЂ![]() ЁЂ
ЁЂ![]() КЭ
КЭ![]() ЫФжждЊЫиФмаЮГЩРызгЛЏКЯЮя
ЫФжждЊЫиФмаЮГЩРызгЛЏКЯЮя
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЩш![]() ДњБэАЂЗќМгЕТТоГЃЪ§ЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ
ДњБэАЂЗќМгЕТТоГЃЪ§ЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ ![]()
![]()
A.![]() жаЃЌЩњГЩ
жаЃЌЩњГЩ![]() БъзМзДПі
БъзМзДПі![]() зЊвЦЕчзгЪ§ЮЊ
зЊвЦЕчзгЪ§ЮЊ![]()
B.56gFeгывЛЖЈСПЕФЯЁЯѕЫсЗДгІЃЌзЊвЦЕчзгЪ§ПЩФмЮЊ![]()
C.зуСПЕФ![]() гывЛЖЈСПЕФХЈбЮЫсЗДгІЕУЕН
гывЛЖЈСПЕФХЈбЮЫсЗДгІЕУЕН![]() ЃЌШєЯђЗДгІКѓЕФШмвКжаМгШызуСПЕФ
ЃЌШєЯђЗДгІКѓЕФШмвКжаМгШызуСПЕФ![]() ЃЌдђЩњГЩAgClЕФЮяжЪЕФСПЮЊ2mol
ЃЌдђЩњГЩAgClЕФЮяжЪЕФСПЮЊ2mol
D.БъзМзДПіЯТЃЌ![]() КЭ
КЭ![]() ЛьКЯКѓЃЌдзгзмЪ§аЁгк
ЛьКЯКѓЃЌдзгзмЪ§аЁгк![]()
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com