
��һ�ְ�ɫ��ĩ���������������Ӻ��������еļ��֡�
�����ӣ�S2����Cl����NO3����SO42����CO32����HCO3����MnO4����
�����ӣ�Na����Mg2����Al3����Ba2����Fe2����Fe3����Cu2����NH4+��
���ð�ɫ��ĩ��������ʵ�飬�۲쵽���������£�
| ʵ����� | ���� |
| a.ȡ������ĩ����ˮ���� | ȫ���ܽ⡢ |
| ��Һ��ɫ�� | |
| b.��������Һ�����������������Һ�������� | ���������� |
| c.ȡ������ĩ�������� | ���������� |
| d.ȡ������ĩ����ϡH2SO4��ϡHNO3�Ļ��Һ | �а�ɫ�������� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����M��һ���ճ������в���ȱ�ٵĵ�ζƷ����֪C����D��ȼ�շ�����ɫ���档M���������ʵ�ת����ϵ����ͼ��ʾ(���ֲ�������ȥ)��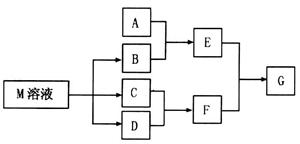
��1��д���ö��Ե缫���M��Һ�����ӷ���ʽ ��
��2����A��һ������������ҿ��������첣������G�Ļ�ѧʽ�� ��
��3����A��һ�ֳ����������ʣ���A��B��Һ�ܹ���Ӧ��������F��Һ��μ���E��Һ���ӱ�����������ʵ�������� ��
��4����A��һ���Σ�A��Һ��B��Һ��ϲ�����ɫ��״������˲���Ϊ����ɫ������ɺ��ɫ��E������Aת����E�����ӷ���ʽ�� ��
��5����A��һ����Һ��ֻ���ܺ���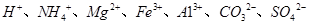 �е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ���Ũ��֮��Ϊ ��
�е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ���Ũ��֮��Ϊ ��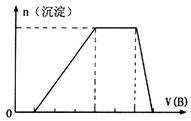
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ֿ���������A��B��C��D��E���������������������ӻ�����ͬ���ֱ�������������K����Fe3����Cu2����Ba2����Al3��������������Cl����OH����NO3-��CO32-��X�е�һ�֡�
��1��ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е�����������________��________��
��2������C�к�������X��Ϊ��ȷ��X���ֽ���1���е��������ʼ�ΪA��B����C��A����Һ���ʱ������ɫ��������ó����е�������ϡHNO3�����������ܽ⣬ʣ���ɫ���塣��XΪ________(����ĸ)��
A��Br�� B��SO42- C��CH3COO�� D��HCO3-
��3����19.2 g CuͶ��װ������D��Һ���Թ��У�Cu���ܽ⣬�ٵμ�ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֣�д��Cu�ܽ�����ӷ���ʽ��________________����Ҫ��Cu��ȫ�ܽ⣬���ټ���H2SO4�����ʵ�����________��
��4�����ö��Ե缫���C��D�Ļ����Һ�����ʵ����ʵ�����Ϊ0.1 mol����������ϵ�л���ͨ��������������������m��ͨ�����ӵ����ʵ���n�Ĺ�ϵ��(������������ֵ)
��5��E��Һ������ᷴӦʱ��������ɫ���ʣ��÷�Ӧ�����ӷ���ʽΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������A��B����ѧ���������ʣ����������ӿɴ��±���ѡ��
| ������ | K����Na����Fe2����Ba2����NH4+ |
| ������ | OH����NO3����I����HCO3����AlO2����HSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D���ֿ��ܵĻ�����������Ӹ�����ͬ�����ֱ���������Na����Mg2����Al3����Ba2����������OH����Cl����SO42-��CO32-������϶��ɡ�Ϊ��ȷ�������ֻ�����ijɷ֣�ijͬѧ����������ʵ�������
�ٽ����ֻ������ȡ���������Һ���ֱ�װ����֧�Թܡ�
��ȡA��Һ�ֱ��������������Һ�У���¼ʵ���������£�
B��Һ ��ɫ����
��ɫ���� �������ܽ�
�������ܽ�
C��Һ ��ɫ����
��ɫ����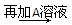 �������ܽ�
�������ܽ�
D��Һ ��ɫ����
��ɫ����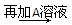 ���������ܽ�
���������ܽ�
����B��Һ�е���D��Һ��������ʵ������
��ش��������⣺
��1��д�����ǵĻ�ѧʽ��A________��B________��C________��D________��
��2�������ڵĵ�����ʵ�飬�ټ���A�����������ܽ�����ӷ���ʽΪ_________________________________________________________________��
��3����������C��Һ�е���D��Һ�����ܳ��ֵ�ʵ��������_________________________________________________________________��
�����ӷ���ʽ��ʾ��ԭ��_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�����Խ����У���MnSO4��Һ��μ�(NH4)2S2O8(���������)��Һ�ᷢ���������ӷ�Ӧ(δ��ƽ)��
Mn2����S2O82����H2O��MnO4����SO42����H����
�ٸ÷�Ӧ�����ڼ���Mn2���Ĵ��ڣ�������������___________________________________��
������Ӧ����0.1 mol��ԭ���μӷ�Ӧ����ת�Ƶ�����Ϊ________NA�����������������ʵ���______________mol��
��д���÷�Ӧ�����ӷ���ʽ_________________________________��
(2)����CuSO4��Һ��ͨ���������ɺ�ɫ����CuS�����ӷ���ʽΪ___________________________________��
����FeCl3��Һ�м�������ĵ⻯����Һ�����ӷ���ʽΪ_____________��
(3)�ڼ��Խ����У�H2O2�н�ǿ�Ļ�ԭ�ԣ�����Ag2O��Ӧ���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ___________________��
(4)Ϊ�ⶨ�����еij���(O3)��������0�桢1.01��105 Pa�Ŀ���V L����ͨ������KI��Һ��ʹ������ȫ��Ӧ��Ȼ��������Һ��a mL c mol��L��1��Na2S2O3��Һ���еζ���ǡ�ôﵽ�յ㡣��֪��2Na2S2O3��I2=Na2S4O6��2NaI��
�ٸõζ������п�ѡ���ָʾ��Ϊ________��
��O3��KI��Һ��Ӧ�������ֵ��ʣ���Ӧ�Ļ�ѧ����ʽΪ___________________________��
�ۿ����г������������Ϊ________(�ú���a��c��V������ĸ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��С�մ���ij������θ������θҩ����Ҫ�ɷ֣���д���÷�Ӧ�����ӷ���ʽ�� ��
����ͨ��ˮ�п��Ƶð�ˮ����ˮ��ʹ��ɫ�ķ�̪��졣��д��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��
���鰱����һ�ֻ�ѧ�����ǣ� ��
��ҩ���й��ġ��Ĵ�����֮һ���ڻ�ҩ�ڷ�����ըʱ���������µķ�Ӧ��2KNO3+C��S��K2S+2NO2��+CO2���������������� ����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ʻش����⣺�� �������ƹ��塡�� ͭ˿���� �Ȼ������塡�� ϡ������Һ���� ������̼���塡�� ���Ǿ��塡�� �����Ȼ���
���ڵ���ʵ��ǣ� �����ţ���ͬ�������ڷǵ���ʵ��� ���ܵ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ȸʯ��һ�ֺ�ͭ�Ŀ�ʯ����ͭ��̬Ϊ
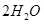 ��ͬʱ����
��ͬʱ����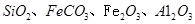 �����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��
�����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��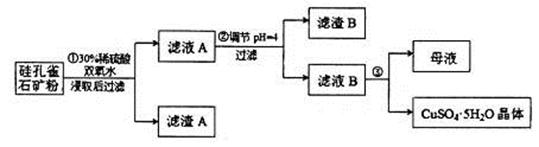
��ش��������⣺
��1����ɲ������ϡ������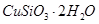 ������Ӧ�Ļ�ѧ����ʽ
������Ӧ�Ļ�ѧ����ʽ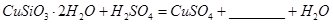 ��
��
�����ӷ���ʽ��ʾ˫��ˮ������_____________________________��
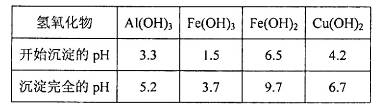
��2������ڵ�����ҺpHѡ�õ�����Լ���__________________
A��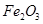 | B��CuO | C��A12O3 | D��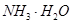 |
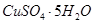 ���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�
���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�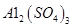 ������Һ��
������Һ�� mol
mol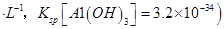 ______________��
______________���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com