
����Ŀ��þ�����������仯�������������������й㷺��Ӧ�á�
��1��þ���Ͻ����ڷɻ�����ҵ������3��90��þ���Ͻ�����������2mol/Lϡ����������0��2mol���������㲢ȷ��þ���Ͻ������ʵ���n(Mg): n(Al)=________��
��2�����������Ҫ�ɷ�ΪFeS2(��������ֻ��SiO2)�����������ԭ�ϡ�ȡij������10g�������Ŀ��������գ�4FeS2+11O2=2Fe2O3+8SO2������ַ�Ӧ����ȴ���Ƶù�������Ϊ7��4g������SiO2����Ӧ��������������FeS2����������Ϊ_____��
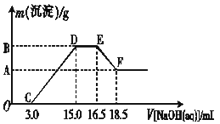
��3������һ���������ۺ�������ɵĻ�����100 mLϡ�����ַ�Ӧ����Ӧ���������κ�����ų�������Ӧ��ij�����Һ������4��00 mol��L��1��NaOH��Һ������NaOH��Һ���������������������Ĺ�ϵ��ͼ��ʾ(��Ҫʱ�ɼ��ȣ�����������ˮ�е��ܽ�)����������A�����ֵ��________��
��4���������������������������֡�ȡij������ĩ28��12g(����ֻ��Fe��C)�����������г�ַ�Ӧ���õ�CO2����224mL(��״����)��
������˸�����ĩ������̼�����ʵ���֮��Ϊ________________����������ȣ���
����ȡ���ݲ�ͬ����������������ĩ�ֱ�ӵ�100mL��ͬŨ�ȵ�ϡH2SO4�У���ַ�Ӧ��õ�ʵ���������±���ʾ��
ʵ����� | �� | �� | �� |
���������ĩ��������g�� | 2��812 | 5��624 | 8��436 |
��������������L������״���� | 1��120 | 2��240 | 2��800 |
���������Һ�����ʵ���Ũ��Ϊ__________________��
����������ʵ�����м�������m�˸�����ĩ�����㷴Ӧ������ʣ��Ĺ�������Ϊ___g(����3λС��)��
���𰸡�1:2 78% 0��856 50��1 1��25 mol/L (m��5��624)��![]() g
g
��������
��1����þ���������ʵ����ֱ�Ϊxmol��ymol�������ݻ�ѧ����ʽ������ɵ�24x+27y=3.9�٣�2x+3y=0.4�ڣ��ɴ˼���þ���Ͻ������ʵ�����
��2���������֪��Ӧǰ�������������10g-7.4g=2.6g����FeS2������Ϊx�����ɻ�ѧ����ʽ�ɵù�ϵʽ480��160=x��2.6g�����x=7��8���ɴ˼�����������FeS2������������
��3���������֪�����ۺ�������ɵĻ�����100 mLϡ�����ַ�Ӧ������������������������泥���ͼ��֪��OC��Ϊ����������������Һ��Ӧ��CD��Ϊ��������������������������Һ��Ӧ����������������������������DE��Ϊ�����������������Һ��Ӧ��EF��Ϊ����������������������Һ����ƫ�����ƣ����յõ���������������
��4���ٱ�״����224mL ������̼�����ʵ���Ϊ![]() =0��01mol����̼ԭ�Ӹ����غ��֪̼�����ʵ���0��01mol���ɸ�����ĩ����Ϊ28��12g��֪�������ʵ���Ϊ
=0��01mol����̼ԭ�Ӹ����غ��֪̼�����ʵ���0��01mol���ɸ�����ĩ����Ϊ28��12g��֪�������ʵ���Ϊ![]() =0��5(mol)���ɴ˼��������ĩ������̼�����ʵ���֮�ȣ�
=0��5(mol)���ɴ˼��������ĩ������̼�����ʵ���֮�ȣ�
���ɱ���֪������������ɷ�Ӧ�������������������������ʵ���Ũ�ȣ�
�۶Ա�ʵ��I��III��ȷ��������ĩ�е���ȫ���ܽ⣬�ɴ˼��㷴Ӧ������ʣ������������
��1����þ���������ʵ����ֱ�Ϊxmol��ymol�������ݻ�ѧ����ʽ������ɵ�24x+27y=3.9�٣�2x+3y=0.4�ڣ���٢ڿɵ�x=0��05��y=0��1����þ���Ͻ������ʵ���n(Mg): n(Al)= 0��05: 0��1=1:2���ʴ�Ϊ��1:2��
��2���������֪��Ӧǰ�������������10g-7.4g=2.6g����FeS2������Ϊx�����ɻ�ѧ����ʽ�ɵù�ϵʽ480��160=x��2.6g�����x=7��8����������FeS2����������Ϊ![]() ��100%/10=78%���ʴ�Ϊ��78%��
��100%/10=78%���ʴ�Ϊ��78%��
��3���������֪�����ۺ�������ɵĻ�����100 mLϡ�����ַ�Ӧ������������������������泥���ͼ��֪��OC��Ϊ����������������Һ��Ӧ��CD��Ϊ��������������������������Һ��Ӧ����������������������������DE��Ϊ�����������������Һ��Ӧ��EF��Ϊ����������������������Һ����ƫ�����ƣ����յõ�����������������ͼ��֪���������ܽ���������������Һ�����Ϊ��18.5��16.5��ml=2ml����3 NaOH��Al��OH��3��NaOH��NaAlO2��֪����������������������Һ�����Ϊ6ml���������������������������������������Ƶ����Ϊ��15.0��3.0��ml=12.0ml���������������������������Ƶ����Ϊ��12.0��6.0��ml=6.0ml����3 NaOH��Fe��OH��3��֪m[Fe��OH��3]=4.0mol/L��0.0060L��![]() ��107g/mol=0��856g����A�����ֵ��0��856���ʴ�Ϊ��0��856��
��107g/mol=0��856g����A�����ֵ��0��856���ʴ�Ϊ��0��856��
��4���ٱ�״����224mL ������̼�����ʵ���Ϊ![]() =0��01mol����̼ԭ�Ӹ����غ��֪̼�����ʵ���0��01mol���ɸ�����ĩ����Ϊ28��12g��֪�������ʵ���Ϊ
=0��01mol����̼ԭ�Ӹ����غ��֪̼�����ʵ���0��01mol���ɸ�����ĩ����Ϊ28��12g��֪�������ʵ���Ϊ![]() =0��5(mol)����˸�����ĩ������̼�����ʵ���֮��Ϊ0��5mol: 0��01mol=50��1���ʴ�Ϊ��50:1��
=0��5(mol)����˸�����ĩ������̼�����ʵ���֮��Ϊ0��5mol: 0��01mol=50��1���ʴ�Ϊ��50:1��
���ɱ���֪�������������״����2��800L���������ʵ���Ϊ![]() = 0��125mol����H2SO4��H2��֪������Һ�����ʵ���Ũ��Ϊ
= 0��125mol����H2SO4��H2��֪������Һ�����ʵ���Ũ��Ϊ![]() =1��25mol/L���ʴ�Ϊ��1��25mol/L��
=1��25mol/L���ʴ�Ϊ��1��25mol/L��
����ǡ���ܽ�ʱ����������Ϊx������ʵ��I��III�����¹�ϵ��![]() �����x��7��030g�������ĸ���������Ϊm��7��030��5��624��1��406g������������ĩ�е���δȫ���ܽ�ʱ����m��1��406g��˵��������ĩ�е���ȫ���ܽ⣬��Ӧ������ʣ����������Ϊ (m��5��624)��
�����x��7��030g�������ĸ���������Ϊm��7��030��5��624��1��406g������������ĩ�е���δȫ���ܽ�ʱ����m��1��406g��˵��������ĩ�е���ȫ���ܽ⣬��Ӧ������ʣ����������Ϊ (m��5��624)��![]() g=(m��5��624)��
g=(m��5��624)��![]() g�����ʴ�Ϊ��(m��5��624)��
g�����ʴ�Ϊ��(m��5��624)��![]() g����
g����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶�ʱ����1molA��2molB�����ݻ�Ϊ5L��ij�ܱ������з������·�Ӧ��A(s)+2B(g)![]() C(g)+2D(g)����5min��ﵽƽ�⣬���������B���ʵ�Ũ�ȼ�����0.2mol��L-1��������������ȷ����
C(g)+2D(g)����5min��ﵽƽ�⣬���������B���ʵ�Ũ�ȼ�����0.2mol��L-1��������������ȷ����
A.��5min�ڸ÷�Ӧ��C��Ũ�ȱ仯��ʾ�ķ�Ӧ����0.02mol��L-1��min-1
B.ƽ��ʱB���������Ϊ33.3%
C.��ʼѹǿ��ƽ��ʱѹǿ��Ϊ4:5
D.ƽ��ʱB��ת����Ϊ50%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��Ӧ�е������仯���ɻ�ѧ��Ӧ�оɻ�ѧ������ʱ���յ��������»�ѧ���γ�ʱ�ų���������ͬ����ġ���ͼΪN2��g����O2��g����Ӧ����NO��g�������е������仯������˵����ȷ����

A.1 mol N2��g����NA�� O2��g����Ӧ�ų�������Ϊ180 kJ
B.1 mol N2��g����1 mol O2��g�������������2 mol NO��g�����������
C.ͨ������£�N2��g����O2��g�������ֱ������NO
D.NO��һ���������������NaOH��Һ��Ӧ�����κ�ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ�к�������NaCl��![]() �����ʵ���֮��Ϊ3:1����ʯī���缫������Һ�����ݵ缫����IJ�ͬ�������Է�Ϊ�����Σ�������������ȷ���ǣ� ��
�����ʵ���֮��Ϊ3:1����ʯī���缫������Һ�����ݵ缫����IJ�ͬ�������Է�Ϊ�����Σ�������������ȷ���ǣ� ��
A.����ֻ����H2B.����������Cl2��������O2
C.�������Ϊ���ˮD.��ҺpH�����������Ϊ7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪���з�Ӧ���Ȼ�ѧ����ʽΪ
��CH3COOH(l)+2O2(g)=2CO2(g)+2H2O(l) ��H1=-870.3kJ��mol-1
��C(s)+O2(g)=CO2(g) ��H2=-393.5kJ��mol-1
��H2(g)+![]() O2(g)=H2O(l) ��H3=��285.8kJ��mol-1
O2(g)=H2O(l) ��H3=��285.8kJ��mol-1
��Ӧ2C(s)+2H2(g)+O2(g)=CH3COOH(l)����HΪ�� ��
A.-488.3kJ��mol-1
B.-191kJ��mol-1
C.-476.8kJ��mol-1
D.-1549.6kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
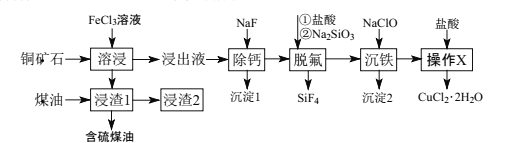
����Ŀ����ͭ��ʯ��Ҫ��������ͭ(Cu2S)��������FeO��CaO��SiO2�ȡ��Ի�ͭ��ʯΪԭ���Ʊ�CuCl2��2H2O�Ĺ���������ͼ��ʾ��
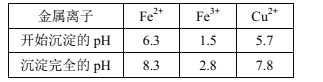
��֪����.��������[c0(Mn+)=0.1mol��L-1]�γ��������������pH��Χ���£�
��.NaCl������ˮ�������ھƾ������¶ȱ仯�ܽ�ȱ仯�����ԣ�CuCl2��H2O������ˮ���ƾ���Ũ��ˮ�����¶������ܽ�ȱ仯��������
��.Ksp(CaF2)=4.0��10-11
��1��Ϊ����ߡ��ܽ���Ч�ʣ��ɲ�ȡ�Ĵ�ʩ��___��д�����ܽ���������Cu2S�ܽ�ʱ���ӷ���ʽ��___��
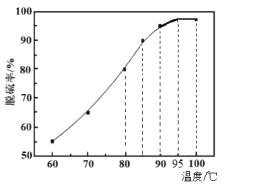
��2��������ʱ�������¶ȵ����ߣ������ʳ��������ƣ���ͼ����ԭ����___��
��3��д�����ѷ���ʱHF������Ӧ�Ļ�ѧ����ʽ��___��
��4��������������������NaClO������������___��
��5��������X���ľ��������___��
��6������������X����ĸҺ����һϵ�в����ɵõ�����һ�����ʣ�Ҫ�õ����ִ�������ѡ�������Լ�ϴ��___��
A��Ũ���� B��ˮ C���ƾ� D��Ũ��ˮ
��7������Һ���м���NaF��ȥ��Һ��Ca2+(Ũ��Ϊ1.0��10-3mol��L-1)������Һ��c(F-)=2.0��10-3mol��L-1ʱ��������Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��̼���γɻ�������������Ԫ�ء�
��CԪ�����γ��л������ҪԪ�أ����з����к���sp��sp3�ӻ���ʽ����___������ĸ����
a.![]() b.CH4 c.CH2=CHCH3 d.CH3CH2C��CH
b.CH4 c.CH2=CHCH3 d.CH3CH2C��CH
��2��BF3����NH3��Ӧ����BF3��NH3��BF3��NH3��Bԭ�ӵ��ӻ��������Ϊ___��B��N֮���γ�___��������(H3BO3)��ˮ��Һ������ˮ��Ӧ����[B(OH)4]-������һԪ��������ʡ�[B��OH��4]-��B��ԭ���ӻ�����Ϊ___�������ǿռ乹�ͣ�[B��OH��4]-�Ľṹ����ʾ��ͼ��ʾΪ___��
��3���������ķ��ֺ�ʹ��������ҽѧʷ��һ���˲���ijɾ͡�
�١�Ц������N2O������������Ӧ����ҽ�Ƶ�������֮һ����֪N2O��������ԭ��ֻ��һ����ԭ����������N2O�ĵ���ʽ�ɱ�ʾΪ___����������幹����___�Ρ�
����һ�ֳ����������ȷ³��治�����������������綾������COCl2����2CHCl3+O2��2HCl+2COCl2��������COCl2�����ӵ����幹����___�Ρ�
��4������ͪ뿳����ڼ���Ni2+����ϡ��ˮ�����У�����ͪ���Ni2+��Ӧ�������ʺ�ɫ��������ṹ��ͼ��ʾ��
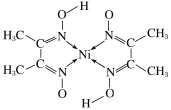
�ٸýṹ�У�̼̼֮��Ĺ��ۼ�������������̼��֮��Ĺ��ۼ�������___������֮���γɵĻ�ѧ����___��
�ڸýṹ�У�̼ԭ�ӵ��ӻ����������___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���0.100mol��L1��NaOH��Һ�ֱ�ζ���Ϊ20.00mL0.100mol��L1��HCl��Һ�ʹ�����Һ���ζ�������ͼ��ʾ������˵����ȷ����
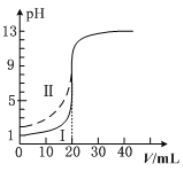
A.���ʾ���ǵζ����������
B.pH=7ʱ���ζ��������ĵ�V��NaOH����20.00mL
C.V��NaOH����20.00mLʱ��������Һ��c��Cl������c��CH3COO����
D.V��NaOH����10.00mLʱ��������c��Na+����c��CH3COO������c��H+����c��OH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʼ�������26�λ��������˰����ӵ�������NA=6.02214076��1023mol-1��������2019��5��20����ʽ��Ч������˵����ȷ���ǣ� ��
A.��Al3+��ĿΪNA��AlCl3��Һ�У�Cl-����Ϊ3 NA
B.�����£�42g��ϩ�ͱ�ϩ�Ļ����������Hԭ�ӵ���Ŀ��6 NA
C.4molNH3��6molO2�ڴ����ͼ��������³�ַ�Ӧ�����ò�����NO�ķ�������4 NA
D.���³�ѹ�£�2g�ǻ���![]() ����������������ΪNA
����������������ΪNA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com