
�о����ʵ��۽ṹ�������������������ʱ仯�ı��ʡ���ش��������⣺
��O��Si��NԪ�صĵ縺���ɴ�С��˳����____________________��C60�ͽ��ʯ����̼��ͬ�������壬�������۵�ϸߵ���____________��
��AΪ�����ڽ���Ԫ�أ������±����ݣ�A�Ļ�̬ԭ�ӵĹ����ʾʽΪ
________________________________��
| ������/kJ��mol��1 | I1 | I2 | I3 | I4 |
| A | 932 | 1821 | 15390 | 21771 |
�ǹ��ɽ���������ˮ�����γɵ�������Ƿ�����ɫ������d����ĵ����Ų��йء�һ��أ���Ϊd0��d10�Ų�ʱ������ɫ����Ϊd1��d9�Ų�ʱ������ɫ����[Cu(H2O)4]2������ɫ���ݴ��ж�25��Ԫ��Mn�γɵ��������[Mn(H2O)6]2��_____(��С����ޡ�)��ɫ��
��H��C��C��COOH�����ں��еĦҼ����м��ĸ�������Ϊ_______________������̼ԭ�ӵ��ӻ���ʽΪ___________________��
��CO������������γ���������Fe(CO)5��Fe(CO)5��һ�������·����ֽⷴӦ��Fe(CO)5(s)��Fe(s)��5CO(g)����Ӧ�����У����ѵĻ�ѧ��ֻ����λ�������γɵĻ�ѧ����������______________��
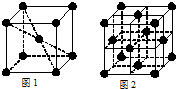
��WԪ�ص�ԭ�ӵ�M�ܲ�Ϊȫ����״̬���Һ����δ�ɶԵ���ֻ��һ����W���������Ķѻ���ʽ����ͼ�� (ѡ��ס������ҡ�����)����W������һ�������ı߳�Ϊa cm����W������ܶ�Ϊ ��д����a�ı���ʽ����NA��ʾ�����ӵ���������
�� �� ��
 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 932 | 1821 | 15390 | 21771 |
| B | 738 | 1451 | 7733 | 10540 |
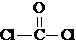 ����COCl2�����ں���
����COCl2�����ں����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2010?���ݶ�ģ��[��ѧ---���ʽṹ������]�о����ʵ��۽ṹ�������������������ʱ仯�ı��ʣ���ش��������⣮
��2010?���ݶ�ģ��[��ѧ---���ʽṹ������]�о����ʵ��۽ṹ�������������������ʱ仯�ı��ʣ���ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �����ܣ�kJ/mol�� | I1 | I2 | I3 | I4 |
| A | 932 | 1821 | 15390 | 21771 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 932 | 1821 | 15390 | 21771 |
| B | 738 | 1451 | 7733 | 10540 |
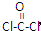 ��COCl2�����ں���
��COCl2�����ں����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ����һ�и߶���ѧ�ڵ�һ���¿���ѧ�Ծ����������� ���ͣ������
��8�֣��о����ʵ��۽ṹ�������������������ʱ仯�ı��ʡ���ش��������⡣
(1)C��Si��NԪ�صĵ縺���ɴ�С��˳����__________________��
(2)A��B��Ϊ�����ڽ���Ԫ�ء������±����ݣ�д��Bԭ�ӵĵ����Ų�ʽ��______________��
| ������/kJ��mol��1 | I1 | I2 | I3 | I4[��Դ:ѧ����ZXXK] |
| A | 932 | 1 821 | 15 390 | 21 771 |
| B | 738 | 1 451 | 7 733 | 10 540 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com