
����Ŀ��Cu3N �������õĵ�ѧ��ѧ�����ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ���̵������У������Ź㷺�ġ���������ľ����á�
(1)C��N��O ����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________________��
(2)��N3-������ͬ����������ԭ�ӷ��ӵĿռ乹����____________��
(3)Cu+�ĵ����Ų�ʽΪ___________������������Һ�в��ȶ����ɷ����绯��Ӧ����Cu2+��Cu,��CuO�ڸ����»�ֽ��Cu2O���Դӽṹ�ǶȽ�������CuO Ϊ�λ�����Cu2O��____________________��
(4)��Cu�Ĵ������£��Ҵ��ɱ���������Ϊ���ᣬ��ȩ������̼ԭ�ӵ��ӻ���ʽ��___________����ȩ����H-C-O�ļ���___________(����ڡ��������ڡ���С�ڡ�)�Ҵ������е�H-C-O �ļ��ǡ�
(5)[Cu(H2O)4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ,�Ի���Cu(H2O)2Cl2���м��Եķ��ӵĽṹʽ��___________��
(6)Cu3N�ľ����ṹ����ͼ��ʾ��N3-����λ��Ϊ___________��Cu+�İ뾶Ϊapm,N3-�İ뾶Ϊbpm,Cu3N���ܶ�Ϊ___________g��cm-3(�����ӵ�������NA��ʾ)��
���𰸡� N>O>C V�� 1s22s22p63s23p63d10(��[Ar]3d10) Cu+��3d����ϵ���ȫ��,��ṹ�ȶ� sp3��sp2�� ���� 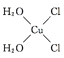 6
6 ![]()
��������(1)ͬ����������ҵ�һ�����ܳ���������,NԪ��ԭ�ӵ�2p�ܼ���3������,Ϊ�����ȶ�״̬,��������,ʧȥ��һ��������Ҫ�������ϸ�,��һ�����ܸ���ͬ��������Ԫ��,�ʵ�һ������N>O>C,��ˣ�������ȷ����: N>O>C;
(2)��N3-������ͬ����������Ϊ�ȵ�����,��NO2-,�õ�����ṹ����,�������������Nԭ�Ӽ۲���ӶԸ���=2+1/2![]() �Һ���һ���µ��Ӷ�,����ΪV�νṹ, ��ˣ�������ȷ����:V��;
�Һ���һ���µ��Ӷ�,����ΪV�νṹ, ��ˣ�������ȷ����:V��;
(3)Cu+�ĺ�����28������,���ݹ���ԭ��֪���̬���Ӻ�������Ų�ʽ1s22s22p63s23p63d10,ԭ�ӹ������ȫ�ա�������ȫ��ʱ���ȶ�, Cu+��3d�����ȫ��,�ȶ�,��ˣ�������ȷ����: 1s22s22p63s23p63d10; Cu+��3d����ϵ���ȫ����ṹ�ȶ�;
(4)��ȩ�����м���̼ԭ�Ӻ���4��![]() ��,ȩ���ϵ�̼ԭ�Ӻ���3��
��,ȩ���ϵ�̼ԭ�Ӻ���3��![]() ��,���Լ��е�̼ԭ�Ӳ���sp3�ӻ�,ȩ���е�̼ԭ�Ӳ���sp2�ӻ�,ȩ����̼ԭ�Ӳ���sp2�ӻ����Ҵ��к��д��ǻ���̼ԭ�Ӳ���sp3�ӻ�,������ȩ������H-C-O�ļ��Ǵ����Ҵ������е�H-C-O�ļ���,��ˣ�������ȷ����: sp3��sp2��;����;
��,���Լ��е�̼ԭ�Ӳ���sp3�ӻ�,ȩ���е�̼ԭ�Ӳ���sp2�ӻ�,ȩ����̼ԭ�Ӳ���sp2�ӻ����Ҵ��к��д��ǻ���̼ԭ�Ӳ���sp3�ӻ�,������ȩ������H-C-O�ļ��Ǵ����Ҵ������е�H-C-O�ļ���,��ˣ�������ȷ����: sp3��sp2��;����;
(5)![]() Ϊƽ�������νṹ,���е�����
Ϊƽ�������νṹ,���е�����![]() ��
��![]() ȡ�������ֲ�ͬ�Ľṹ,
ȡ�������ֲ�ͬ�Ľṹ,![]() ���м��Եķ���,˵���÷��ӵĽṹ���Գ�,����ṹʽΪ
���м��Եķ���,˵���÷��ӵĽṹ���Գ�,����ṹʽΪ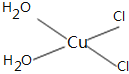 ��
��
��ˣ�������ȷ����: ��
��
(6)![]() �ľ����ṹ��ͼ,�������
�ľ����ṹ��ͼ,�������![]() ,С�����
,С�����![]() ,���Դ����ʾCuԭ�ӡ�С���ʾNԭ��,
,���Դ����ʾCuԭ�ӡ�С���ʾNԭ��,![]() ����λ��
�����![]() ,���������
,���������![]() ,
,![]() ���ܶ�
���ܶ�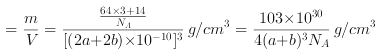 ,
,
��ˣ�������ȷ����:6;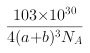 .
.



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1������ʽΪC7H8�ķ����������Ĵ��������������ӳɺ����ɻ���������д���û�������һ�ȴ���Ľṹ��ʽ��______��______��_______��_______��_______��
��2������C8H18��һ��ͬ���칹��ֻ����һ��һ�ȴ����д�������칹��Ľṹ��ʽ___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�����A������KAl(SO4)2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��

�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ�������ȡ�ķ��뷽����___________________��
��2������������ͼ��Ӧ��ϵ��д������B��C��E�������ʵĻ�ѧʽ
��������B________________��C______________________��E_______________
��3��д���١����ĸ���Ӧ����ʽ�������ӷ�Ӧ��д���ӷ���ʽ��
��____________________________ ��__________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������SO2(g)��O2(g)�ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У��ڲ�ͬ�¶��½��з�Ӧ��2SO2(g)+ O2(g)![]() 2SO3 ��H<0���õ�����е���������������˵������ȷ����
2SO3 ��H<0���õ�����е���������������˵������ȷ����
ʵ���� | �¶�/�� | ƽ�ⳣ��/mol-1��L | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
SO2 | O2 | SO2 | O2 | ||||
1 | T1 | K1 | 4 | 2 | x | 0.8 | 6 |
2 | T2 | K2 | 4 | 2 | 0.4 | y | t |
A. T1��T2�Ĺ�ϵ��T1 �� T2
B. x= 1.6��y=0.2 ��t<6
C. K1��K2�Ĺ�ϵ��K2��K1
D. ʵ��1��ǰ6min�ķ�Ӧ������(SO2)=0.2 mol��L-1��min-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijģ�����˹���Ҷ���绯ѧʵ��װ����ͼ��ʾ����װ���ܽ�H2O��CO2ת��ΪO2��ȼ��(C3H8O)������˵����ȷ����( )
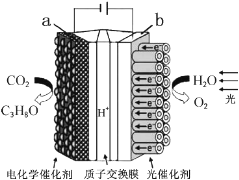
A. ��װ�ý���ѧ��ת��Ϊ���ܺ͵���
B. ��װ�ù���ʱ��H+��b������a����Ǩ��
C. ÿ����1molO2����44g CO2����ԭ
D. a�缫�ķ�ӦΪ��3CO2+18H+-18e-=C3H8O+5H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Fe3O4��һ�ֺ�ɫ��ĩ���ֳƴ�����������������ɿ�д��FeO��Fe2O3��ij��ѧʵ��С��ͨ��ʵ����̽��һ��ɫ��ĩ�Ƿ���Fe3O4��CuO���(������������ɫ����)��̽���������£�
��������裺 ����1����ɫ��ĩ��CuO������2����ɫ��ĩ��Fe3O4;
����3����ɫ��δ��CuO��Fe3O4�Ļ����
�����̽��ʵ�飺
����һ��ȡ������ĩ��������ϡ���ᣬ������2�����3������ʵ��������___________����ط�Ӧ�����ӷ���ʽΪ__________________________________��
�������� �������ϣ� Cu2+��������ˮ��Ӧ��������ɫ��Һ��Cu2++4NH3��H2O=Cu(NH3)42++4H2O��Ϊ̽���Ǽ���2���Ǽ���3��������ȡ������ĩ��ϡ�������ܽ���ټ���������ˮ��������___________���������2������������___________���������3������
��������
ѧ����������ͼ��ʾװ�ý���ʵ�飬������Ӧǰ��װ��C����Ʒ����������ȷ����Ʒ����ɡ��ش��������⣺
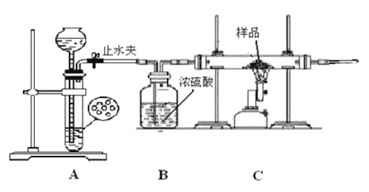
(1)����ʵ�鲽����Ⱥ�˳����___________(�����)��
�ٴ�ֹˮ�У��ڹر�ֹˮ�У��۵�ȼC���ľƾ���ƣ�
��Ϩ��C���ľƾ���� ���ռ��������鴿��
�ڵ�ȼC���ƾ����ǰҪ���еı�Ҫ������__________________________��
(2)������Ʒȫ���μӷ�Ӧ����ʵ��ǰ��Ʒ������Ϊ4.7�ˣ�ʵ���Ƶ�װ��C�й��������3.6�������___________(�1����2����3��) ��ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I���������а�������: ���� ������ ��SiO2 ��H2SO4 ��NaOH ��FeSO4��Һ ��Ba(OH)2 ��������������
(1)�����������ڵ���ʵ���____________(�����)���ܵ������__________ (�����)��
(2)����м���ٵķ�ĩ����Ӧ�����ӷ���ʽΪ_____________________��
(3)����������������������ˮ��Һ�з�Ӧ�����ӷ���ʽΪH++OH-=H2O����÷�Ӧ�Ļ�ѧ����ʽΪ__________________________��
(4)ʵ�����Ʊ�������ӷ���ʽΪ:__________________�����ڢ��л�������ܵ���Һ��������������________________________��
(5) ���Dz�������Ҫ�ɷ�֮һ���������Һ��Ӧ�Ļ�ѧ����ʽΪ____________________������ʦ����_______(����������)����̲�����
II�����и������ʵķ�����ᴿ��Ӧѡ��������������һ��?(��ѡ����ĸ)
A.��Һ B.���� C.��ȡ D.���� E.�����ᾧ F.���·ֽ�
(1)����CCl4��H2O:___________��
(2)��ȥ����ʯ��ˮ��������CaCO3: ___________��
(3)����CCl4(�е�Ϊ76.75��)�ͼױ�(�е�Ϊ110.6��)��Һ������:__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���� �����ӷ���ʽ��д��ȷ����( )
A. ����ͨ��ˮ�У�Cl2��H2O = 2H����Cl����ClO��
B. ����ϡ���ᷴӦ��2Fe��6H�� = 2 Fe3����3H2��
C. ̼�������ᷴӦ��CaCO3��2H�� = Ca2����CO2����H2O
D. ��������������������Һ��Ӧ��Al(OH)3��OH�� = AlO2����2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��д��ȷ����
A. ��FeCl3��Һ��ʴͭ��·�壺Cu + Fe3+ �� Cu2+ + Fe2+
B. ���Ȼ�����Һ�м�������İ�ˮ��Al3+ + 4NH3��H2O = AlO2- + 4NH4+ + 2H2O
C. Na2O2������H2O��Ӧ����O2 ��2Na2O2 + 2H2O �� 4Na+ + 4OH�� + O2��
D. ������KMnO4��Һ��ͨ��SO2��3SO2��2MnO��4OH��== 2MnO2����3SO![]() ��2H2O
��2H2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com