
����Ŀ��п�ڹ�ҵ������Ҫ���ã�Ҳ������������Ԫ�ء��ش��������⣺
��1��Znԭ�Ӻ�������Ų�ʽΪ______________��
��2����ͭ����������ʹ�õĺϽ�֮һ����Ҫ��Zn��Cu��ɡ���һ��������1��Zn��_______��1��Cu)(����ڡ���С�ڡ�)��ԭ����_____________��
��3��ZnF2���нϸߵ��۵㣨872 ��)���仯ѧ��������________��ZnF2�������л��ܼ���ZnCl2��ZnBr2��ZnI2�ܹ������Ҵ������ѵ��л��ܼ���ԭ����______________��
��4�����л����ݡ�����ҽ�伮�У�������¯��ʯ��ZnCO3����ҩ������������Ƥ����֢����洴�ˡ�ZnCO3�У������ӿռ乹��Ϊ____��Cԭ�ӵ��ӻ���ʽΪ______��
��5������Zn�����е�ԭ�Ӷѻ���ʽ��ͼ��ʾ�����ֶѻ���ʽ��Ϊ_______��

���𰸡�[Ar]3d1 04s2����Zn��������Ų�Ϊȫ���ȶ��ṹ������ʧ���ӣ����Ӽ�ZnF2Ϊ���ӻ����ZnCl2��ZnBr2��ZnI2�Ļ�ѧ���Թ��ۼ�Ϊ�������Խ�Сƽ��������sp2�������ܶѻ�
��������
��1��Zn�ǵ�30��Ԫ�أ����������Ų�ʽΪ [Ar]3d1 04s2����2��Zn�ĵ�һ�����ܴ���Cu�ĵ�һ�����ܣ���ΪZn����Χ����ÿ���ܼ�������ȫ����״̬���ʴ�Ϊ�����ڡ�Zn��������Ų�Ϊȫ���ȶ��ṹ������ʧ��������3���ɷ���п���۵���ж���Ϊ���ӻ�����ʴ������Ӽ������ӻ�����һ���������л��ܼ��У����Ȼ�п���廯п���⻯пΪ���ۻ�������Ӽ��Խ�С���ܹ������Ҵ��������Ե��л��ܼ��У��ʴ�Ϊ��ZnF2Ϊ���ӻ����ZnCl2��ZnBr2��ZnI2�Ļ�ѧ���Թ��ۼ�Ϊ�������Խ�С����4��̼��п�е�������ΪCO32-�����ݼ۲���ӶԻ������ۣ�������ԭ��C�ļ۵��Ӷ�Ϊ3+(4��3��2��2)/2=3�ԣ����Կռ乹��Ϊ�������Σ�����CΪsp2�ӻ����ʴ�Ϊ��ƽ����������sp2����5����ͼʾ���ѻ���ʽΪ�������ܶѻ����ʴ�Ϊ���������ܶѻ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��˫���绯ѧ��(װ����ͼ)���ڴ�ͳ���װ���з����˵����Ե缫BPE��ͨ��ʱ��BPE���˽���������Ʋ�����ݶȺϽ������й�˵���������

A.mΪ��Դ����
B.BPE��b�˱�a�˵ĵ��Ƹ�
C.BPE��a�˷����ķ�ӦΪ�� 2H2O+2e-=H2��+2OH-
D.BPE��b�˵����ĵIJ�ͬλ�ã����γ���ɲ�ͬ��ͭ���Ͻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ҫ�Ļ���ԭ�ϣ���ҽҩ��ұ�𡢻�����ʳƷ�����㷺ʹ�á�ijʵ��С���ͬѧģ���°��Ƽ��ȡ����������£�

��1����ҵ��������ĵ�һ���dz�ȥ����ʳ��ˮ����SO42-��Ca2+���ӣ����μ�����Լ�����������______________��_______________�����ˡ�_______________��
��2����֪�������ε��ܽ��
NaCl | NH4HCO3 | NaHCO3 | NH4Cl | |
�ܽ�ȣ�20��C��100gH2Oʱ�� | 36.0 | 21.7 | 9.6 | 37.2 |
��д��װ��I�з�Ӧ�Ļ�ѧ����ʽ_____________________________________��
��д��װ��II�з�����Ӧ�Ļ�ѧ����ʽ________________________________��
��3���������п�ѭ�����õ�������__________________��
��4�����Ƴ��Ĵ�����ֻ��������NaCl��
�ټ����øô������Ƶ���Һ�к���Cl�D�ķ�����_________________________��
����m�˴�����Ʒ�м�������CaCl2��Һ�����������ˡ�ϴ�ӡ������������Ϊb g����ô���Ĵ��ȣ�����������Ϊ__________����m��b����ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�����I��II������Ӧ����ͬһ��Ӧ���͵��ǣ� ��
ѡ�� | ��ӦI | ��ӦII |
A |
| CH2=CH2��CH3CH2Cl |
B | CH3CH2Cl��CH3CH2OH | CH3CH2OH��CH3COOCH2CH3 |
C | CH3CH2OH��CH2=CH2 | CH3CH2OH��CH3CHO |
D | ��֬������ |
|
A. AB. BC. CD. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��300����ǰ��������ѧ�Ҳ����������������ûʳ�������ɫ��Ӧ�����ɴ˷���������īˮ��ûʳ����Ľṹ��ʽΪ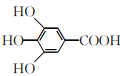 �����й���ûʳ�����˵����ȷ����
�����й���ûʳ�����˵����ȷ����
A. ����ʽΪC7H7O5
B. �ܷ����ӳɷ�Ӧ����ȥ��Ӧ��ȡ����Ӧ
C. 1 molûʳ������������̼�����Ʒ�Ӧ������4 mol CO2
D. 1 molûʳ�������������Ʒ�Ӧ������2 mol H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ժ���ͭ��CuO��Cu��Ϊԭ���Ʊ��Ȼ���ͭ��CuCl����һ�ֹ����������£�
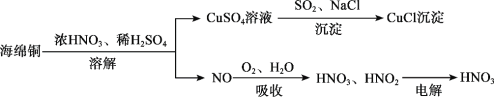
��1��Cu��̬ԭ�Ӻ�������Ų�ʽΪ________��SO42 �Ŀռ乹��Ϊ__________����������������Cu2+ ��OH�� ��Ӧ������[Cu(OH)4] 2��[Cu(OH)4] 2 ���ṩ�µ��ӶԵ�ԭ����_______����Ԫ�ط��ţ���
��2�������ա����̣�
��2NO(g) + O2(g)![]() 2NO2(g) ��H =��112.6kJmol1���NOƽ��ת���ʵķ�����______________��д�����֣���
2NO2(g) ��H =��112.6kJmol1���NOƽ��ת���ʵķ�����______________��д�����֣���
������NO2���йط�Ӧ���£�
��Ӧ��2NO2(g) + H2O(l) �� HNO3(aq) + HNO2(aq) ��H =��116.1kJmol1
��Ӧ��3HNO2(aq) �� HNO3(aq) + 2NO(g) + H2O(l) ��H =��75.9kJmol1
��ˮ����NO2����HNO3��NO���Ȼ�ѧ����ʽ��___________________��
��3������⡱���̣�HNO2Ϊ���ᣬͨ�����ʹHNO3���������������ĵ缫��Ӧʽ��____________��
��4�������������̣�����CuCl�����ӷ���ʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��36.5g�Ȼ����ܽ���1Lˮ��(ˮ���ܶȽ���Ϊ1g��mL��1)��������Һ���ܶ�Ϊ��g��mL��1����������Ϊw�����ʵ���Ũ��Ϊcmol��L��1��NA��ʾ�����ӵ�������ֵ��������������ȷ����(����)
A. ������Һ�����ʵ���Ũ��Ϊ1mol��L��1B. 36.5g�Ȼ��⺬��NA��ԭ��
C. 36.5gHCl����ռ�е����ԼΪ22.4LD. ������Һ����������w��36.5c/1000��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״���£����������壺��6.72 L CH4����3.01��1023��HCl���ӣ���13.6g H2S����0.2 mol NH3�������йرȽ�����ȷ����
A.���������>��>��>��
B.����������>��>��>��
C.��ԭ����Ŀ���٣���>��>��>��
D.Ħ�������Ĵ�С����>��>��>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧϰС��������µ�ʵ�鷽�����ⶨþ���Ͻ�����������������
����һ����m1gþ���Ͻ����ܽ����������Լ�X�У���ַ�Ӧ���ˡ�ϴ�ӡ�����������õ�����Ϊm2 g�Ĺ��塣
��1���Լ�X��_____����Ӧ�����ӷ���ʽ��____��
��2�������ʵ��Ĺ����У�û�жԹ������ϴ�ӣ�������������������_____������ƫ������ƫС��������Ӱ��������
��������ѡ����ͼ��ʾ��װ�ú�ҩƷ��

��3����װ�������Ժ���Ҫ���е�ʵ�������_____��
��4������a��������______��
��5������ʱ��Ҫע���������_____��
��6������Ͻ��������a g�������������Ϊb L���ѻ���Ϊ��״��ʱ�����������Ͻ�����������������_____��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com