
����Ŀ��[���ʽṹ������]
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ��������ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��1��ԭ������ͬ���۵�����Ҳ��ͬ��������Ϊ�ȵ����塣�ȵ�����������ƵĻ�ѧ���������������ơ�CO�ĽṹʽΪ_______________��
��2��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣬д����̬��ԭ�ӵĺ�������Ų�ʽ______ ��
��3������ϩ���������ھ������õĹ�����ܣ���̫���ܵ�ص�Ӧ���Ͼ��зdz�������ǰ;������ϩ(![]() )�Ľṹ��ͼ��������̼ԭ�ӹ�����ӻ�����Ϊ______��1 mol
)�Ľṹ��ͼ��������̼ԭ�ӹ�����ӻ�����Ϊ______��1 mol ![]() ������
������![]() ������ĿΪ______ ��
������ĿΪ______ ��
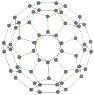
��4����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�ء����ӡ���п��ͭ������Ĥ��صȡ�
�ٵ�һ�����ܣ�As_____Se(����>������<������=��)��
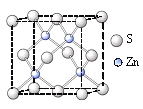
����п�ľ�����(�ṹ����ͼ��ʾ)�������ӵ���λ����_______��
�۶����������ӵĿռ乹��Ϊ________��
��5������̪ݼ������ڹ�̫���ܵ��������Ҫ���ã�һ�ֽ���þ̪ݼ�����Ľṹ����ͼ��������ͼ���ü�ͷ��ʾ����λ����________________
���𰸡�![]() 1s22s22p63s23p63d84s2 sp2 90NA �� 4 ���ͣ����͡�V�ͣ�
1s22s22p63s23p63d84s2 sp2 90NA �� 4 ���ͣ����͡�V�ͣ� 
��������
��1��CO��N2��Ϊ�ȵ����壬����N2�Ľṹʽ��дCO�Ľṹʽ��
��2����ԭ�Ӻ�����28�����ӣ����ݹ���ԭ����д���̬��ԭ�ӵĺ�������Ų�ʽ��
��3���ж�̼ԭ�ӵ��ӻ����Ϳɸ�����ɼ�������жϣ�̼ԭ�������ĸ����������ӻ����ͱ�Ϊsp3������һ��˫�����������������ӻ����ͱ�Ϊsp2������һ��������һ�����������ӻ����ͱ�Ϊsp�������þ�̯��������������Ŀ��
��4����ͬһ����Ԫ�صĵ�һ����������ԭ�����������������������ƣ���ע���VA��Ԫ�ش���ͬ��������Ԫ�صĵ�һ�����ܣ�
���������������п���ӵ���ĿΪ�����ӵ���λ����
�ۼ���Seԭ�ӹµ��Ӷԡ��۲���Ӷԣ�ȷ����ռ乹�ͣ�
��5����λ�����ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ�ӡ�
��1���ȵ�����������ƵĻ�ѧ��������CO��N2��Ϊ�ȵ����壬��ṹʽ���ƣ���CO�ĽṹʽΪC![]() O��
O��
�ʴ�Ϊ��C![]() O��
O��
��2����ԭ�Ӻ�����28�����ӣ����̬��ԭ�ӵĺ�������Ų�ʽΪ��1s22s22p63s23p63d84s2����
�ʴ�Ϊ��1s22s22p63s23p63d84s2��
��3����C60������ÿ��̼ԭ����Χ�γ�һ��˫����������������ÿ��̼ԭ���γ�3���������Ҳ����µ��Ӷԣ����Բ���sp2�ӻ���ÿ��̼ԭ�Ӻ��е���������Ϊ![]() ������1molC60��������������Ŀ=1mol��60��
������1molC60��������������Ŀ=1mol��60��![]() ��NAmol1=90NA��
��NAmol1=90NA��
�ʴ�Ϊ��sp2��90NA��
��4����As��Se����ͬһ���ڣ���As���ڵ�VA�壬Se���ڵ�VIA�壬Asԭ��4p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���Se��
�ʴ�Ϊ��>��
���������������п���ӵ���ĿΪ1��4=4���������ӵ���λ����4��
�ʴ�Ϊ��4��
�۶�������������Seԭ�ӹµ��Ӷ���Ϊ![]() ���۲���Ӷ���=2+1=3��������ռ�ṹΪV�Σ�
���۲���Ӷ���=2+1=3��������ռ�ṹΪV�Σ�
�ʴ�Ϊ�����ͣ����͡�V�ͣ���
��5����λ�����ṩ�µ��Ӷ�ԭ��ָ���ṩ�չ����ԭ�ӣ����Ը�������е���λ��Ϊ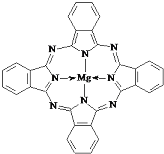 ��
��
�ʴ�Ϊ�� ��
��


 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�Ƿ�������ʱ���õ����������Խ��еĻ�����������ֱ��ǣ���
![]()
A.�����ˡ���ȡ������B.������������ȡ������
C.��ȡ�����ˡ���������D.���ˡ���������ȡ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ϊ����һ�������������仯�������������������й㷺��;��
��1����ԭ����Χ���ӹ����ʾʽΪ____________________����ԭ�Ӻ�����ӷ���ԾǨʱ�����ջ��ͷŲ�ͬ�Ĺ⣬������_________��ȡ��Ԫ�ص�ԭ�ӹ��ס�
��2��FeCoOx��һ���������������ĵ����ܴ�С��ϵ��I4(Co)____I4(Fe)������>������<�� ) ��ԭ����_____________��
��3����ï��[(C5H5)2Fe]���������ȼ�����Ӽ������Ϳ���������ï���۵�172�棬�е�249 �棬��������������ˮ���������л��ܼ���������_____________���塣
��4�������ϩ��C5H6���ṹ��ͼ��a�����������ƶ�ï���������ϩ��̼ԭ�ӵ��ӻ���ʽΪ________�������еĴ��������÷���![]() ��ʾ����m���������γɴ�������ԭ������n���������γɴ������ĵ������������ϩ�����ӣ�C5H5�D���ṹ��ͼ(b)�����еĴ��������Ա�ʾΪ_________________��
��ʾ����m���������γɴ�������ԭ������n���������γɴ������ĵ������������ϩ�����ӣ�C5H5�D���ṹ��ͼ(b)�����еĴ��������Ա�ʾΪ_________________��
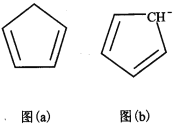
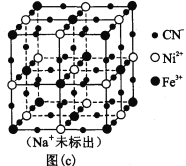
��5��ij��³ʿ������������Ϊ���������ӵ�ص缫���ϡ�����Na+��Ni2+��Fe3+��CN�D���ɣ��侧���ṹ��ͼ(c)���������У�������________�����ţ���A�����Ӽ� B�� ���� C�� ���� D�� ��� E�� ������
��6���þ�����Fe3+���õĶѻ���ʽ��_______��ѡ��Po��Na��Mg��Cu����ͬ��������������λ��϶�й�����_____�� Na+��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��������̬���ʣ���һ���¶�����仯������ͼ������˵��һ����ȷ���ǣ� ��
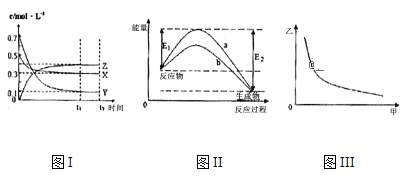
A.�÷�Ӧ���Ȼ�ѧ����ʽΪX(g) +3Y(g) 2Z(g) ��H= -��E2-E1��kJ
B.��ͼIII�мױ�ʾѹǿ���ұ�ʾZ�ĺ���������仯����ͼIII������
C.���¶��£���Ӧ��ƽ�ⳣ����ֵԼΪ533���������¶ȣ��÷�Ӧ��ƽ�ⳣ����С��Y��ת���ʽ���
D.ͼII������b�Ǽ������ʱ�������仯���ߣ�����a��û�м������ʱ�������仯����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[��ѧ����ѡ��ѧ�뼼��]
�������������������ش��й����⡣
(1)ij��������������ķֲ������ͼ��ʾ��������W��X��Y��Z��ij��ˮ������������ʾ��
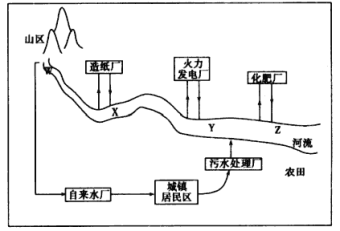
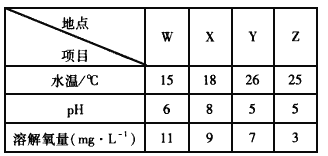
�ٵ���X��Y��ˮ��pH�仯��ԭ�������______________________________��
��Z������������٣��������������ԭ�������________________________��
(2)ij������̽���̲��зḻ�ij�������Ҫ�ɷ�ΪFe2O3��������SiO2�����ʣ���ú��ʯ��ʯ����������ڸõ������������������
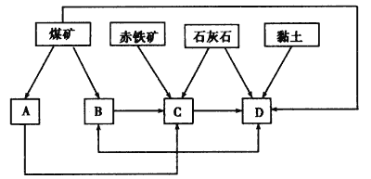
����������Ŀ������������Ľ�������Ҫ�ڸõ�����Ӧ���������������糧��ˮ�೧�ȣ��γɹ�ģ�Ĺ�ҵ��ϵ���ݴ�ȷ����ͼ����Ӧ����������A__________��B__________��C__________��D__________��
���Գ�����Ϊԭ�ϣ�д����¯�����еõ������Ͳ���¯���Ļ�ѧ����ʽ__________��
�۴������������á����������ȽǶȿ��ǣ��õ�������ҵ��������Ӧ��ȡ��һЩ��ʩ�У��ٳ�2�ִ�ʩ���ɣ�_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͬѧ����Ϥ�����ʣ�
��O2����H2O2����MgCl2����H2SO4����Na2CO3��NH4Cl����CO2����Ne����Na2O2����NaOH
(1)��Щ�����У�ֻ���й��ۼ�����__________________________________(����ţ���ͬ)��ֻ�������Ӽ�����___________________________���Ⱥ��м��Թ��ۼ��ֺ������Ӽ�����_____________________________���Ⱥ��зǼ��Թ��ۼ��ֺ������Ӽ�����________________________�����ڻ�ѧ������_________________________________��
���ڹ��ۻ��������_________________________________��
(2)д���������ʵĽṹʽ
��O2 _______________��H2O2_______________��CO2_______________
(3)д���������ʵĵ���ʽ
��NH4Cl ______________��Na2O2 _______________����NaOH_______________
(4)�õ���ʽ��ʾ�������ʵ��γ�
��O2_______________________________________________________________
��MgCl2____________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йذ�����ε�˵������ȷ����
A. ����ʪ��ĺ�ɫʯ����ֽ���鰱��
B. ������ζ���ˮ������Һ������ʱ��c(NH4+) = c(Cl)
C. NH3�����������
D. ����ʱ��0.1 mol��L1NH4Cl��Һ��ˮϡ�ͣ�![]() ��ֵ����
��ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���⼰�仯�����ڿ��С�����ȷ����й㷺��;����ͼ��ʾΪ�Ӻ�������ȡ��Ĺ������̡�
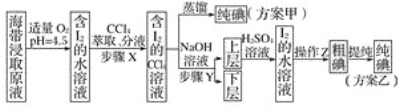
��֪��3I2��6NaOH===5NaI��NaIO3��3H2O��
��ش��������⣺
��1����Һ©��ʹ��ǰ��Ҫ��©����©����Ϊ__________��
��2������X�У���ȡ���Һ©���ڹ۲쵽��������_____________��
��3�������йز���Y��˵������ȷ����_____(����ĸ)��
A��Ӧ����NaOH��Һ��Ũ�Ⱥ����
B�����ⵥ��ת���ɵ����ӽ���ˮ��
C����Ҫ�dz�ȥ������ȡԭҺ�е��л�����
D��NaOH��Һ�������Ҵ�����
��4�������ϲ����м���H2SO4��Һ��������Ӧ�Ļ�ѧ����ʽΪ____������Z������Ϊ________��
��5�������������������������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(As)�뵪����ͬһ���壬�����γ�As4��As2S3��As2O5��H3AsO3��H3AsO4�����ʣ����Ź㷺����;���ش��������⣺
��1��Asԭ���������ӵĵ�������չ������___�֡�����(As4)�����(P4)�Ľṹ���ƣ����¹��ڻ�������ıȽ�������ȷ����___(����)��
A��������۵���ڰ��� B�������й��ۼ����ܴ��ڰ���
C��������Ӽ��Դ��ڰ��� D�������й��ۼ����Ǿ�Ϊ109��28��
��2��AsԪ�صķǽ����Ա�N������ԭ�ӽṹ��֪ʶ˵�����ɡ�___��
��3��298Kʱ����20mL3xmol/LNa3AsO3��20mL3xmol/LI2��20mLNaOH��Һ��ϣ�������Ӧ��AsO33-(aq)+I2(aq)+2OH-(aq)![]() AsO43-(aq)+2I-(aq)+H2O(l)����Һ��c(AsO43-)�뷴Ӧʱ��(t)�Ĺ�ϵ��ͼ��ʾ��
AsO43-(aq)+2I-(aq)+H2O(l)����Һ��c(AsO43-)�뷴Ӧʱ��(t)�Ĺ�ϵ��ͼ��ʾ��
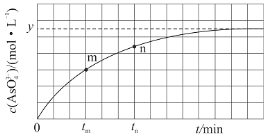
����ƽ��ʱ��pH=14���÷�Ӧ��ƽ�ⳣ��Ϊ___��
�ڵ���Ӧ�ﵽƽ��ʱ������ѡ����ȷ����__�����ţ���
a����Һ��pH���ٱ仯 b��v(I-)=2v(AsO33-)
c��![]() ���ٱ仯 d��c(I-)=ymol/L
���ٱ仯 d��c(I-)=ymol/L
��tmʱ��v��__v����������������С������������������
��tmʱ��v��__tnʱv����������������С������������������������__��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com