
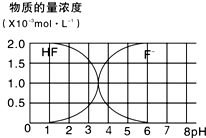
 ��1����������HA��������NaA�Ļ����Һ���ڻ�ѧ������������Һ�������м�����������ʱ����Һ������Ա仯�����ֽ�0.04mol?L-1HA��Һ��0.02mol?L-1NaOH��Һ�������ϣ��õ�������Һ��
��1����������HA��������NaA�Ļ����Һ���ڻ�ѧ������������Һ�������м�����������ʱ����Һ������Ա仯�����ֽ�0.04mol?L-1HA��Һ��0.02mol?L-1NaOH��Һ�������ϣ��õ�������Һ��

 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д� ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ϩ�����ȵ��������ص�ˮ��Һ |
| B�������Ҵ������ȵ��������ص�ˮ��Һ |
| C��������ϩ������170���½��е� |
| D�������Ҵ������ȵ��������صĴ���Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ڢۢ� | B���ڢ� |
| C���ڢۢ� | D��ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����Գ���ˮ��AOW������������HlNl��������Ϊ��������ǿ������ |
| B��ˮ�Ĵ������õ�Ư�ۺ����������ߵ�����ԭ����ͬ |
| C�����������������Լ��������������������������Ҫ��� |
| D���ع��͵���Ҫ�ɷ�����֬������������͡�ú�Ͳ���ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c ��HCN����c ��CN-�� |
| B��c ��Na+����c ��CN-�� |
| C��c ��HCN��-c ��CN-��=c ��OH-�� |
| D��c ��HCN��+c ��CN-��=0.1mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H+ |
| ��ת�� |
| Fe2+ |
| �ڻ�ԭ |
| OH- |
| �۳��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
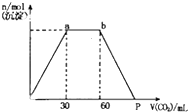 ��Ba��OH��2��KOH���Һ�л���ͨ��CO2���������������ɳ������ʵ�����ͨ��CO2�������VmL�Ĺ�ϵ��ͼ��ʾ�����н�������ȷ���ǣ�������
��Ba��OH��2��KOH���Һ�л���ͨ��CO2���������������ɳ������ʵ�����ͨ��CO2�������VmL�Ĺ�ϵ��ͼ��ʾ�����н�������ȷ���ǣ�������| A��ԭ�������n[Ba��OH��2]��n��KOH��=1��2 |
| B��p������Ϊ120mL |
| C��p����Һ������ΪBa��HCO3��2 |
| D��a��b�η�Ӧ�ֶ��Σ����ӷ���ʽΪ��CO2+2OH-=CO32-+H2O CO32-+H2O+CO2=2HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| C |
| D |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com