
����Ŀ��ʵ����Ҫ����480mL0.1mol��L-1Na2CO3��Һ���ش��������⡣
��1��Ӧ��������ƽ��ȡNa2CO3��10H2O����___g��
��2������Na2CO3��Һʱ���õ���Ҫ�����������ձ�����������___��___��
��3����ʵ���������������Һ��Ũ��ƫ�͵���___
A����ˮʱ�����̶���
B�����ǽ�ϴ��Һ��������ƿ
C������ƿ�ڱڸ���ˮ���δ���и��ﴦ��
D�����ݺ�ҡ�ȣ�Һ����ڿ̶���
E������ʱ��������
���𰸡�14.3g 500ml����ƿ ��ͷ�ι� AB
��������
��1������n=cv��������Na2CO3�����ʵ���������Na2CO310H2O�����ʵ�������Na2CO3�����ʵ���������m=nM����Na2CO310H2O��������
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ��������������
��3������c=![]() ���㲻��������n��V��Ӱ�죬���nƫ���VƫС������������ҺŨ��ƫ�ߡ�
���㲻��������n��V��Ӱ�죬���nƫ���VƫС������������ҺŨ��ƫ�ߡ�
��1����������Һ�����Ϊ480mL��������ƿ�Ĺ��û��480mL��ֻ��ѡ��500mL��
Na2CO3�����ʵ���n=cV=0.5L��0.1molL-1=0.05mol��Na2CO310H2O�����ʵ�������Na2CO3�����ʵ���������Na2CO310H2O������0.05mol��286g/mol=14.3g��
��2������˳���ǣ��������������ܽ⡢��ȴ����Һ��ϴ����������ҡ����װƿ��ǩ��һ������ƽ�������õ�ҩ�ף����������ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��3��A����ˮʱ�����̶��ߣ���Һ�����ƫ��Ũ��ƫ�ͣ�ѡ��A��ȷ��
B�����ǽ�ϴ��Һת������ƿ��ϴ��Һ�к������ʣ����ʵ�����ƫС��Ũ��ƫ�ͣ�ѡ��B��ȷ��
C������ƿϴ�Ӻ�ײ��ڱ���ˮ���δ�����ﴦ������Ӱ����Һ�������Ũ�Ȳ��䣬ѡ��C����
D�����ݡ�ҡ�ȡ����ú��ְ�Һ����ڿ̶��ߣ�����������ڿ̶������ϵ���Һ�ͻ����䣬��Һ��������䣬Ũ����Ӱ�죬ѡ��D����
E������ʱ�������⣬���ƹ�������ƫ��������ҺŨ��ƫ��ѡ��E��ȷ��
��ѡAB��


 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ0.1mol/LNaOH��Һ450mL��������Һ����������ش��������⣺
��1����ͼ��ʾ�������У�������Һ�϶�����Ҫ����____������ţ�������������Һ�����õ��IJ���������____�����������ƣ���
��2�����ݼ��㣬��������ƽ��ȡNaOH������Ϊ___g����ʵ����������������ȷ��������ƿ������ˮϴ�Ӻ�δ�����������ҺŨ��____0.1mol/L������ڡ���С�ڡ����ڡ�����ͬ��������δ����Һ��ȴ�Ͷ��ݣ���������ҺŨ��___0.1mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ�п��ܺ���OH-��CO32-��A1O2-��SiO32-��SO42-��HCO3-��Na+��Fe3+��Mg2+��Al3+�����ӡ��������Һ����μ���һ�����ʵ���Ũ�ȵ�������Һʱ���������ɳ��������ʵ�����������Һ������仯����ͼ��ʾ������˵����ȷ����

A. ԭ��Һ�п��ܺ���Na2SO4�����ܲ�����CO32-
B. ԭ��Һ��һ�����е�������ֻ��:OH-��A1O2-��CO32-
C. ԭ��Һ�к�CO32-��A1O2-�����ʵ���֮��Ϊ3:4
D. a-d>3/4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̽��������̼�ں�����ת�ƺ��ޣ��ǵ������ѧ�о���ǰ�������о����������ں�ˮ�Ķ�����̼��Ҫ����̼��ʽ���ڣ�����HCO3-ռ95%����ѧ��������ͼ��ʾװ�ôӺ�ˮ����ȡCO2�������ڼ��ٻ����������庬����

����˵������ȷ����
A. a����OH-�ڵ缫���ϱ�����
B. b�ҷ�����Ӧ�����ӷ���ʽΪ��H+ + HCO3- = CO2�� + H2O
C. ��·��ÿ��0.2mol ����ͨ��ʱ������0.2mol�����Ӵ�c������b��
D. ��������ȼ�ϵ�ع��磬���ظ������ܷ����ķ�ӦΪ��H2 + 2OH- - 2e- =2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪X��Y��Z��Ϊ��ѧ��ѧ�������ʣ��Ҿ�����ͬһ��Ԫ�أ�����X�ǵ��ʣ�����֮���ת����ϵ��ͼ��ʾ����X��Y��Z�������ǣ� ��
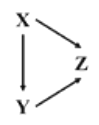
���� ѡ�� | X | Y | Z |
A | Na | Na2O | NaOH |
B | Fe | FeCl3 | FeCl2 |
C | Mg | Mg(OH)2 | MgO |
D | Si | SiO2 | Na2SiO3 |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��

��֪����4FeO��Cr2O3+8Na2CO3+7O2![]() 8Na2CrO4+2Fe2O3+8CO2����
8Na2CrO4+2Fe2O3+8CO2����
��Na2CO3+Al2O3![]() 2NaAlO2+CO��
2NaAlO2+CO��
���������գ�
��1���������ڳ����¸÷�Ӧ���ʼ��������д�ʩ����ʹ��Ӧ�����������____��
A.�����¶� B.ͨ������Ŀ��� C.��ԭ�Ϸ��� D.���Ӵ��������
��2������X����Ҫ����___����д��ѧʽ����
��3�����������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ����___��____��ϴ�ӡ����
��4�������������ʵ��ܽ�����ݣ��������з�����Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7+2KCl=K2Cr2O7��+2NaCl���÷�Ӧ����Һ���ܷ�����������____������˵������
���� | �ܽ�ȣ�g/100gˮ�� | ||
0�� | 40�� | 80�� | |
KCl | 28 | 40.1 | 51.3 |
NaCl | 35.7 | 36.4 | 38 |
K2Cr2O7 | 4.7 | 26.3 | 73 |
Na2Cr2O7 | 163 | 215 | 376 |
��5������ƷY��Ҫ��������������������þ���������ܻ����P���������ʣ���ȷ����Y���������������ķ����dz�ȡ![]() ��Ʒ���������____����д�Լ������ܽ⡢���ˡ��ټ��루��ͨ�룩____����д�Լ������������ա���ȴ���������ø������
��Ʒ���������____����д�Լ������ܽ⡢���ˡ��ټ��루��ͨ�룩____����д�Լ������������ա���ȴ���������ø������![]() ��������Ʒ��������������������Ϊ___���ú�m��n�Ĵ���ʽ��ʾ����
��������Ʒ��������������������Ϊ___���ú�m��n�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F�������ʵ�ת����ϵ��ͼ��ʾ(��Ӧ�����Ͳ��ֲ���δ���)��

��1����AΪ�����ڽ������ʣ�DΪ�����ڷǽ������ʣ�������Ԫ�ص�ԭ������A��D��2��������Ԫ�ص�ԭ������������D��A��2����F��Ũ��Һ��A��D��Ӧ���к���ɫ�������ɣ���A��ԭ�ӽṹʾ��ͼΪ________����Ӧ�ܵĻ�ѧ����ʽΪ____________________________________________________��
��2����A�dz����ı�۽����ĵ��ʣ�D��F����̬���ʣ��ҷ�Ӧ����ˮ��Һ�н��С���Ӧ��Ҳ��ˮ��Һ�н��У������ӷ���ʽ��_____________________________________ ����֪����������D��F��Ӧ����B��д���÷�Ӧ�Ļ�ѧ����ʽ��______________________________________
��3����A��D��F���Ƕ����ڷǽ���Ԫ�ص��ʣ���A��D����Ԫ��ͬ���壬A��F����Ԫ��ͬ���ڣ���Ӧ�ٵĻ�ѧ����ʽΪ_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������£�NO��NH3���Է�����Ӧ����N2��H2O������NO��NH3�Ļ����1mol����ַ�Ӧ�����ò����У�������ԭ�õ���N2�Ⱦ������õ���N2��1.4g��
��1��д����Ӧ�Ļ�ѧ����ʽ���������ת�Ƶķ������Ŀ______________��
��2�������Ϸ�Ӧ������ȫ���Լ���ԭ��Ӧ�������NO��NH3�����ʵ������ܸ���____��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ƽ���з���ʽ����˫���ŷ���ʾ������ת�Ƶ���Ŀ�� Fe2O3 + KNO3 + KOH = K2FeO4 + KNO2 + H2O ��____________�������� ________ ���������� __________
��2���õ����ŷ���ʾ����������ԭ��Ӧ����ϵ, ���������ת�Ƶ���Ŀ����MnO2�����ܶ�Ϊ1.19 g/cm3��������������Ϊ36.5%��HCl��Һ����Ӧ�õ�������Һ����Ӧ�Ļ�ѧ����ʽΪ�� MnO2+4HCl��Ũ��![]() MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
�ٸ÷�Ӧ�����ӷ���ʽΪ�� _____________________________________________������������ʵ���Ũ��Ϊ___________mol/L��
���������뻹ԭ�����ʵ���֮��Ϊ_______________
��8.7gMnO2������Ũ���ᷴӦ��ʹ________molHCl�����������У���ԭ����Ϊ��________��_______��__________��
������÷�Ӧת��1mol���ӣ������ɱ�״���µ��������Ϊ___________L��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com