
����Ŀ���������(HN3)������(NaN3��NH4N3��CuN3��) �����б�ը�ԣ�����Ͼ�������ѧ�����ɽ����Ŷӳɹ��ϳɳ�PHAC���仯ѧʽΪ(N3)3 (NH4)4Cl���ش����⣺
(1) PHAC����N3���Ļ��ϼ�Ϊ_____________��N2F2�ĵ���ʽΪ_____________��
(2)������ȫ������NaN3�ɷ������з�Ӧ��
NaN3(s)=Na(s)+![]() N2(g) ��H1
N2(g) ��H1
2NaN3(s)+CuO(s)=Na2O(s)+3N2(g)+Cu(s) ��H2
��ӦCuO(s)+2Na(s)= Na2O(s)+ Cu(s) ��H=_______(�á�H1�͡�H2��ʾ)
(3) 25�棬��1molNH4N3(s)Ͷ��һ2L�ĺ����ܱ������У�0.5min��Ӧ�ﵽƽ�⣬������ɵ����ֵ��ʵ����ʵ���֮��Ϊ1.6mol����NH4N3��ƽ��ת����Ϊ________��25��ʱ�÷�Ӧ��ƽ�ⳣ��K=__________��
(4) �������(HN3)������ˮ������������������
�� HN3��ˮ��Һ�еĵ��뷽��ʽΪ_____________��
�� 0.1mol��L-1��HN3��Һ��0.1mol��L-1��NaN3�������ϣ������Һ�и�����Ũ���д�С��˳��Ϊ_________________��
�� ��֪T��ʱ��Ksp(CuN3)=5.0��10-9��Ksp(Cu2S)=2.5��10-48������ͬ�¶��·�Ӧ��
Cu2S(s)+2N3-(aq) ![]() 2CuN3(s)+S2-(aq)��ƽ�ⳣ��K=_______________��
2CuN3(s)+S2-(aq)��ƽ�ⳣ��K=_______________��
���𰸡���1 ![]() H2 -2H1 40% 0.0256 HN3
H2 -2H1 40% 0.0256 HN3![]() H++N3- c(N3-)>c(Na+)>c(H+)>c(OH-) 1.0��10-31
H++N3- c(N3-)>c(Na+)>c(H+)>c(OH-) 1.0��10-31
��������
(1)���ݵ�����ԭ������(N3)3(NH4)4Cl����N3���Ļ��ϼۣ�����ԭ�������ﵽ8�����ȶ��ṹ�Ĺ���N2F2�ĵ���ʽ��
(2)���ݸ�˹���ɼ�������Ӧ���ʱ䣻
(3)NH4N3(s)������Ӧ�������ֵ��ʣ�ӦΪN2��H2�����ݷ�Ӧ����ʽ����ԭ�ϵ�ƽ��ת���ʣ�����ƽ�ⳣ������ʽ����÷�Ӧ��25��ʱ��ƽ�ⳣ��K��ֵ��
(4)��HN3�ǵ����ᣬΪ������ʣ���ˮ��Һ�е����H+��N3-���ݴ�д�����뷽��ʽ��
�ڵ�����������������ƣ�0.1mol/L��HN3��Һ��0.1mol/L��NaN3�������ϣ���ҺӦ��HN3����Ϊ������Һ�����ԣ��ݴ˱Ƚ���Һ������Ũ�ȵĴ�С��
�۸��ݶ���ƽ�������㷴Ӧ����ʽ��ƽ�ⳣ����
(1)���ݵ�����ԭ�����㻯�ϼۣ�PHAC�Ļ�ѧʽΪ(N3)3 (NH4)4Cl������Cl��-1�ۣ�NH4)��+1�ۣ�����N3���Ļ��ϼ�ӦΪ-1�ۣ�ԭ�������һ����ﵽ8�����ȶ��ṹ����N2F2�ĵ���ʽӦΪ��![]() ���ʴ�Ϊ��-1��
���ʴ�Ϊ��-1��![]() ��
��
(2)��NaN3(s)�TNa(s)+![]() N2(g)��H1����2NaN3(s)+CuO(s)�TNa2O(s)+3N2(g)+Cu(s)��H2�����ݸ�˹���ɣ���ӦCuO(s)+2Na(s)�TNa2O(s)+Cu(s)���ɢ�-2���ٵõ�������H=��H2-2��H1���ʴ�Ϊ����H2-2��H1��
N2(g)��H1����2NaN3(s)+CuO(s)�TNa2O(s)+3N2(g)+Cu(s)��H2�����ݸ�˹���ɣ���ӦCuO(s)+2Na(s)�TNa2O(s)+Cu(s)���ɢ�-2���ٵõ�������H=��H2-2��H1���ʴ�Ϊ����H2-2��H1��
(3)NH4N3(s)������Ӧ�������ֵ��ʣ�ӦΪN2��H2����Ӧ����ʽΪ��NH4N3(s)2N2(g)+2H2(g)��25�棬��1molNH4N3(s)Ͷ��һ2L�ĺ����ܱ������У�0.5min��Ӧ�ﵽƽ�⣬������ɵ����ֵ��ʵ����ʵ���֮��Ϊ1.6mol�����ɵ����ֵ������ʵ�����ȣ���Ϊ0.8mol�����ݷ�Ӧ����ʽ���ֽ�����ʵ���Ϊ0.4mol����ƽ��ʱNH4N3�����ʵ���Ϊ0.6mol������ԭ�ϵ�ת����Ϊ��=![]() ��100%��40%���������ΪV=2L����ƽ��ʱ�����Ũ��Ϊ��c(N2)=c(H2)=
��100%��40%���������ΪV=2L����ƽ��ʱ�����Ũ��Ϊ��c(N2)=c(H2)=![]() =0.4mol/L����25��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��ΪK=c2(H2)c2(N2)=(0.4mol/L)2��(0.4mol/L)2=0.0256mol4/L4���ʴ�Ϊ��40%��0.0256mol4/L4��
=0.4mol/L����25��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��ΪK=c2(H2)c2(N2)=(0.4mol/L)2��(0.4mol/L)2=0.0256mol4/L4���ʴ�Ϊ��40%��0.0256mol4/L4��
(4)���������(HN3)������ˮ������������������������������������ʣ���ˮ��Һ�е����H+��N3-����HN3��ˮ��Һ�еĵ��뷽��ʽΪ��HN3H++N3-���ʴ�Ϊ��HN3H++N3-��
�ڵ�����������������ƣ�0.1mol/L��HN3��Һ��0.1mol/L��NaN3�������ϣ���ҺӦ��HN3����Ϊ������Һ�����ԣ�����Һ��c(N3-)��c(Na+)��c(H+)��c(OH-)��������Һ�и�����Ũ���д�С��˳��Ϊ c(N3-)��c(Na+)��c(H+)��c(OH-)���ʴ�Ϊ��c(N3-)��c(Na
��Cu2S(s)+2N3-(aq)2CuN3(s)+S2-(aq)���÷�Ӧ�Ļ�ѧƽ�ⳣ��ΪK=![]() =
=![]()
![]() =
=![]() =
=![]() =1.0��10-31���ʴ�Ϊ��1.0��10-31��
=1.0��10-31���ʴ�Ϊ��1.0��10-31��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£�pH=11�İ�ˮ��NaOH��Һ�ֱ��ˮϡ��100������Һ��pH����Һ����仯����������ͼ��ʾ����ͼ�жϴ������(�� ��)

A. a����ֵһ������9
B. ��Ϊ��ˮϡ��ʱ��Һ��pH�仯����
C. ϡ�ͺ�ˮ��ˮ�ĵ���̶ȱ�NaOH��Һ��ˮ�ĵ���̶ȴ�
D. ��ȫ�к���ͬ���������Һʱ��������ͬŨ�ȵ�ϡ����������V(NaOH)<V(��ˮ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����в���ǰ������Ԫ�ص����ʻ�ԭ�ӽṹ���±���

(1)B���ʷ����У�����________��![]() ����__________��
����__________��![]() ����Ԫ��B����̬�⻯��Ŀռ���Ϊ________________��
����Ԫ��B����̬�⻯��Ŀռ���Ϊ________________��
(2)C���ʵ��۵�____________A���ʵ��۵�(��������������������)����ԭ���ǣ�_______________
(3)д��Ԫ��D��̬ԭ�ӵĵ����Ų�ʽ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ԭ��ص����������Է�Ӧ��Fe+Cu2+=Fe2++Cu���һ��ԭ��أ�����ʾ��ͼ�����ԭ��ص����������͵��ӵ������ڷ����У���_________________
��ѡ�õĵ������Һ�У�ϡ���ᡢ����ͭ��Һ
��ѡ�õĵ缫�����У�пƬ��ͭƬ����Ƭ
�缫���ϼ��缫��Ӧʽ��
�������ϣ�______________���缫��Ӧʽ��___________________��
�������ϣ�______________���缫��Ӧʽ��___________________��
�������Һ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ï��������ȼ�ϵĽ������̼����������ȡ�ʵ�����Ʊ���ï��װ��ʾ��ͼ��ͼһ
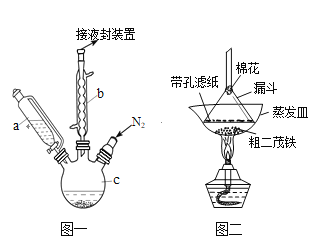
��֪���ٶ�ï���۵���173������100��ʱ��ʼ�������е���249����
���Ʊ���ï���ķ�Ӧԭ���ǣ�2KOH+FeCl2+2C5H6= Fe(C5H5)2+2KCl+2H2O
ʵ�鲽��Ϊ��
����������ƿ�м���25g��ĩ״��KOH����������a�м���60mL��ˮ���ѵ���ƿ�У���ֽ��裬ͬʱͨ����Լ10min��
���ٴ�����a����5.5mL������Ļ����ϩ��C5H6, �ܶ�Ϊ0.95g/cm3�������裻
�۽�6.5g��ˮFeCl2��(CH3)2SO���������������ܼ�����ɵ���Һ25mLװ������a�У�������������c�У�45min���꣬��������45min��
���ٴ�����a����25mL��ˮ���ѽ��裻
�ݽ�c�е�Һ��ת���Һ©���У����������ᡢˮ��ϴ�����Σ���Һ�óȻ�ɫ��Һ��
�������Ȼ�ɫ��Һ���ö�ï���ֲ�Ʒ��
�ش��������⣺
��1������b��������________________________.
��2���������ͨ�뵪����Ŀ����____________________________________________________.
��3������c�������ݻ�ӦΪ(ѡ���)��_________
��100mL ��250mL ��500mL
��4�������������ϴ�ӵ�Ŀ����__________________________________________________
��5��������Ƕ�ï���ֲ�Ʒ���ᴿ���ù�����ͼ���н��У����������Ϊ_________���ò���������������______________________________________________________.
��6��Ϊ��ȷ�ϵõ����Ƕ�ï��������Ҫ���е�һ���ʵ����__________________________���������Ƶô����Ķ�ï��4.3g�����ʵ��IJ���Ϊ____________��������λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ͭ�������A�ṹ��ͼ����ش��������⣺

(1)д����̬Cu����Χ�����Ų�ʽ_____________________________________��
(2)���就�������(H2NCH2COO��)���ȷֽ�ɲ���CO2��N2��N2��������������Ŀ֮����__________��N2O��CO2��Ϊ�ȵ����壬��N2O�ĵ���ʽΪ____________��
(3)��Cu���£��״��ɱ�����Ϊ��ȩ����ȩ������HCO�ļ���_____(ѡ������������������������С����)120������ȩ����ˮ�γ������������ͼ�б�ʾ����_____��

(4)������������ͼ����ʯ�ṹ���ƣ��dz�Ӳ���ϡ���������������B-N��������ԭ����֮��Ϊ___________��

(5)Cu����Ķѻ���ʽ��ͼ��ʾ����Cuԭ�Ӱ뾶Ϊa��������Cuԭ�ӵ���λ��Ϊ________������Ŀռ�������Ϊ______��(��֪:![]() ����ʽ����������)
����ʽ����������)

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�HNO2�ĵ���ƽ�ⳣ��ΪK��4.6��10-4(��֪![]() 2.14)����20mL0.01molL��1HNO2��Һ����μ�����ͬŨ�ȵ�NOH��Һ����û��Һ�� pH��NaOH��Һ����ı仯��ͼ��ʾ�������ж���ȷ����
2.14)����20mL0.01molL��1HNO2��Һ����μ�����ͬŨ�ȵ�NOH��Һ����û��Һ�� pH��NaOH��Һ����ı仯��ͼ��ʾ�������ж���ȷ����

A. X=20
B. a��b��c��d�ĵ��Ӧ����Һ��ˮ�ĵ���̶���С
C. a����Һ��c(H��)��2.14��10��3molL��1
D. b����Һ����Ũ�ȵĴ�С��ϵΪc(HNO2)��c(Na+)��c(NO2��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ɡ����(ͼI)������Ⱦ�ϡ�ҽҩ�����ϵ��м��塣����˵���������
A. ���¶�ɡ������Һ̬��������ˮ
B. ͼ�����ʵ�һ�ȴ�����5�ֽṹ
C. ��ɡ���������9��̼ԭ�ӹ�ƽ��
D. ͼ���⻯��Ӧ���Ǽӳɷ�Ӧ���ǻ�ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������֤�����������̼������Ӧ����ȷ��������װ����ͼ��ʾ(��֪��PdC12��Һ��CO�ܲ�����ɫ��Pd)������˵���������

A. װ�âٵ�������������ȡH2��NH3������
B. װ�â���ʯ��ˮ����Ǻ��ٵ�ȼ�ƾ���
C. װ�âڢ��зֱ�ʢװ����Na2CO3��Һ��ŨH2SO4
D. װ�â����к�ɫ�����������ķ�Ӧ��PdC12+CO+H2O=Pd��+CO2+2HC1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com